MYOCHIP | H2020 European project
Introduction
Myochip: Building a 3D innervated and irrigated muscle on a chip
The aim of the European Union’s Horizon H2020 project, Myochip (https://myochip.imm.medicina.ulisboa.pt) is to build a muscle on chip. Myochip gathers 4 partners from all around the world: The institute of molecular medicine (Portugal), The University of Edinburg (Scotland), and Institut Curie (France).
Seeding cells in hydrogels: the benefit of pressure based flow controllers
Organs on chips are considered as the next generation in vitro models to replace traditional 2D cell culture both in fundamental science and in drug development. This technology focuses on reproducing the microenvironment of cells at the cellular level:
- The intercellular interaction by positioning one cell type relative to another
- The extracellular matrix by reproducing both its composition and its microarchitecture
- The local mechanical constraints by reproducing the shear stress or the local motion of cells
There are very few, if any models available that reproduce all these features. Most of them focus on one aspect to induce a more physiological phenotype to cells.
In this context, Myochip aims to reproduce both the extracellular matrix and the spatial positioning of the 3 different cell types that constitute muscle: muscle cells, endothelial cells and neurons.
The first part of the project is dedicated to the reproduction of muscle fibres. As they differentiate muscle cells fuse to form a syncytium. Their nuclei are positioned on the external part of the fibre and the cytoskeleton organize to form a network of actin and myosin that controls muscle contractility. Muscle fibres have a typical size of a hundred microns in diameter and a few millimetres in length.
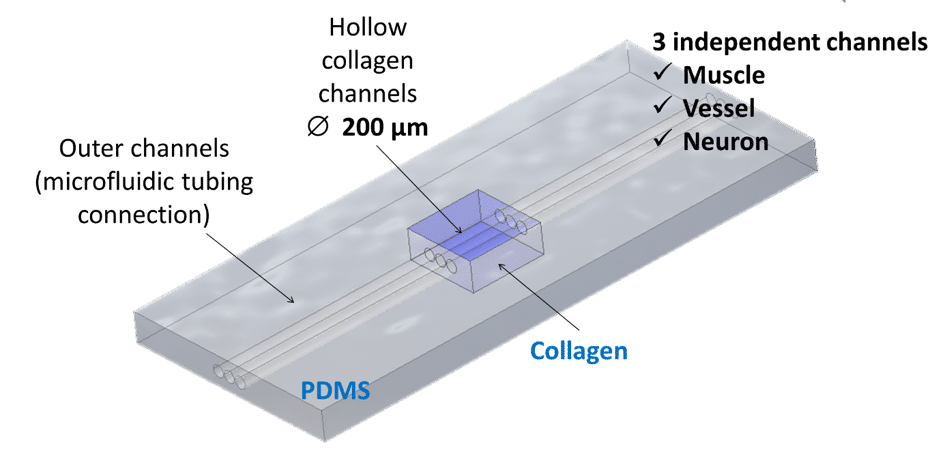
To induce cellular self-organization, Institut Curie first textured the collagen I to create hollow cylindric channels of 200µm in diameter spaced 200µm apart inside the collagen bulk. Muscle cells are then seeded inside. This allows the cells to adhere, proliferate, grow and organize into myofibers. Besides the complexity of the chip architecture in structuring the scaffold, another challenge is in the seeding itself. In typical chips made out of polymers, cells are seeded manually with a pipette. With hydrogels, however, the seeding should be finely controlled. Hydrogels are highly fragile and the non-controlled pipetting on cells leads to hydrogel distortions and even to hydrogel detachment from the chip.
Movie and image courtesy of Manh Louis Nguyen, MMBM team , Institut Curie
To overcome this issue, a Flow-EZ pressure controller was used to gently inject cells inside the collagen channels. The pulse free injection preserves the architecture of the 3D hydrogel and promotes improved cell survival when compared to the acute shear stress associated with manual pipetting. The difference between these two modes of injection are illustrated in the videos below: