A mRNA encapsulation platform integrating Fluigent’s FlowEZ
Due to the impact of the coronavirus (COVID 2019) outbreak in 2019, mRNA Vaccines emerged as a frontrunner in vaccinology, with the 2023 Nobel Prize of Medicine being awarded to Dr. Katalin Karikó and Dr. Drew Weissman for their work development in this field. mRNA vaccines present simple and rapid manufacturing compared to traditional vaccines with a reduced production cost. The production process can be streamlined and automated, which offers the opportunity to develop rapidly in mRNA technology for both research and industrial scale.
In this paper from Microsystem & Nanoengineering (2023, 9:97), Hongjuan Wei et al. established a universal integrated platform for the streamlined and on-demand preparation of mRNA products directly from DNA templates. This platform includes a microfluidic mRNA encapsulation module using Fluigent pressure-driven controllers (FlowEZ) and flow sensors (FlowUnit).
A Bioinformatics Center of AMMS (Beijiing) Paper
Paper: Wei, H.; Rong, Z.; Liu, L.; Sang, Y.; Yang, J.; Wang, S. Streamlined and On-Demand Preparation of mRNA Products on a Universal Integrated Platform. Microsyst Nanoeng 2023, 9 (1), 97. https://doi.org/10.1038/s41378-023-00538-8.
mRNA vaccines, a game-changer in vaccinology
Traditional vaccines are made either of a weakened form of the virus or viral proteins. In contrast, mRNA vaccines only include the instruction needed for the cell to produce a small part of the virus, and thus teach our immune system to recognize it.
Without any risk of gene insertion as injected mRNA are translated into proteins in the cell cytoplasm, these types of vaccines have been proven to be long-lasting and effective with safe immune responses.1,2
In addition, a key advantage of mRNA vaccines is their simple and rapid manufacturing compared to traditional vaccines, especially since they can be produced in a laboratory without any cell culture requirement.
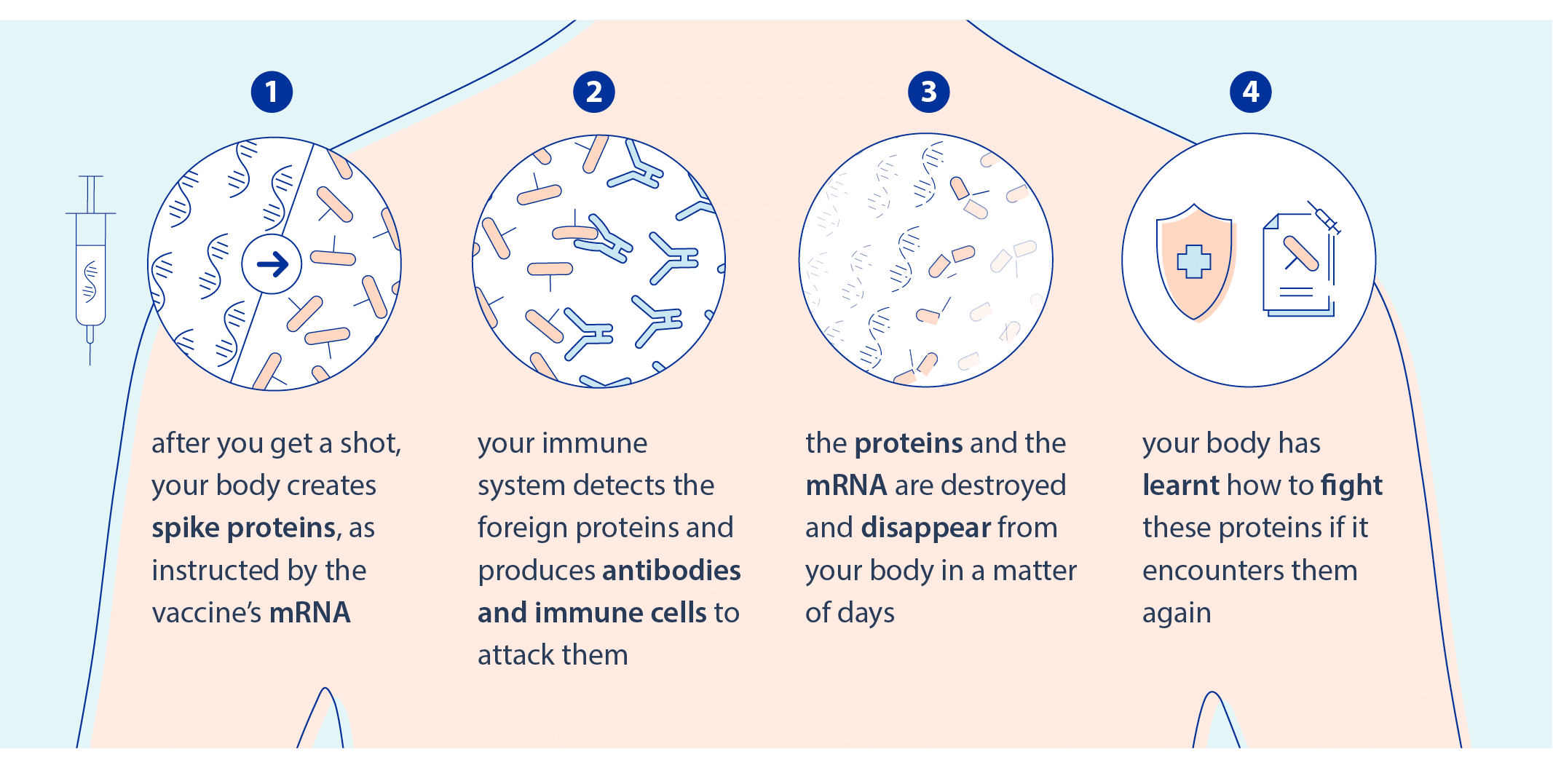
Figure 1: Mechanism of mRA vaccine in the body after injection.3
How can microfluidics help in developing mRNA vaccines?
Before protecting us from the disease, mRNA should be protected from being destroyed in its free state by chemicals present in our blood and enzymatic degradation during the vaccine injection. To overcome this issue, lipid nanoparticles (LNP) are used as shields through mRNA encapsulation. This approach ensures the circulation of mRNA through the blood toward the targeted cell type. Nonetheless, this process requires homogeneous size and distribution of the lipid nanocarriers, which are key parameters in determining clinical success.
The traditional method, which often consists of slowly injecting the lipid solution into an aqueous phase, lacks control over the encapsulation efficiency and the size of the lipid nanocarriers. In contrast, microfluidics offers the opportunity to master these parameters with high reproducibility, easy automation, and a streamlined process.
In this perspective, Hongjuan Wei et al. developed an automated “on-demand” mRNA preparation platform, which contains a module dedicated to encapsulating mRNA in lipid nanoparticles using a microfluidic approach. This example of a universal platform could be a powerful tool in terms of rapidly generating mRNA treatment to accelerate the progress for the early research and development stage.
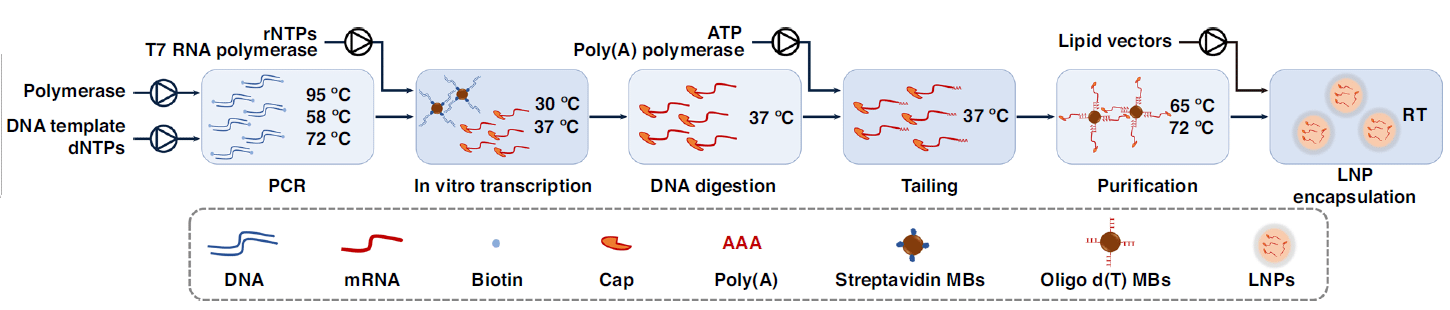
The platform included three main components: a polymerase chain reaction (PCR) module to amplify the target DNA templates, a heating–magnet separating–mixing module to provide a mixing platform for in vitro transcription, and the encapsulation module to directly encapsulate mRNA in LNPs. The performance of this platform was evaluated by starting with an enhanced green fluorescent protein (eGFP).
Herein, we will focus on the procedure and results for encapsulating mRNA in lipid nanoparticles through the developed module.
A microfluidic encapsulation module for mRNA LNP
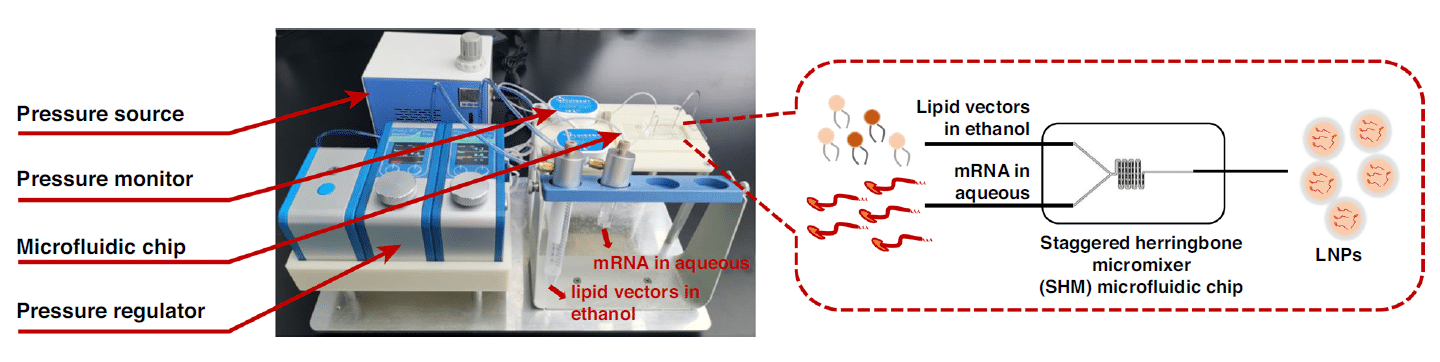
The microfluidic encapsulation technique used in this work is based on a staggered herringbone micromixing chip, Fluigent’s pressure-driven controllers (FlowEZ), and flow sensors (FlowUnit). By pressurizing two separate microfluidic reservoirs that contain the lipid vectors in ethanol and eGFP mRNA in water respectively, the fluids circulate toward the microfluidic chip.
Two FlowUnits (Range L: 0-1000 µL/min) were installed for measuring the flow during the process, and thus regulating the pressure through the FlowEZs. The design of the chip consisted of a Y-junction, that enabled the integration of the two fluids, followed by a serpentine channel where a micromixing occurred and enhanced through the herringbone shapes.
The obtained mRNA-LNP was observed by TEM, analyzed by DLS, and further evaluated by transfection into HEK-293T cells.
Partial results: Stable, small, and monodisperse mRNA lipid nanocarriers
Prior to the mRNA encapsulation module, eGFP was successfully used to develop a group of workflows in the overall platform, including washing, self-testing, PCR amplification, in vitro transcription with capping, DNA digestion, poly(A) tailing, and purification.
Regulated with Fluigent’s pressure-driven controllers (FlowEZ) and flow sensors (FlowUnit), the flow rates for the lipid vectors in ethanol and for the mRNA in water were 300 μL/min and 900 μL/min respectively.
Through the staggered herringbone chip which provided an induced mixing of the two fluids, the LNPs were formed by electrostatic interaction when the lipid vectors dissolved in ethanol diffused and mixed with a nucleic acid solution. The hydrodynamic diameter of the produced LNPs, analyzed by DLS, revealed an average size of 111.1 ± 2.08 nm.
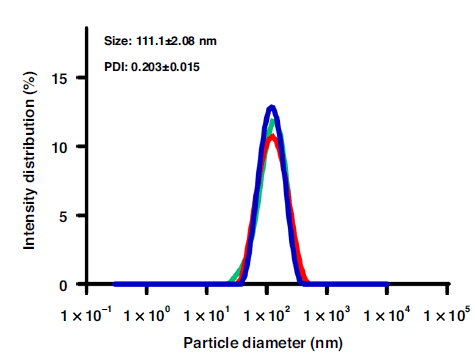
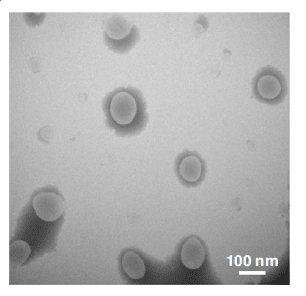
In addition, TEM images revealed the spherical shape of the LNPs and confirmed their size, which was in accordance with the DLS analysis. These results show the possibility of forming relatively small and stable LNPs with a low variation in diameter.
This is helped by the constant flow regulated by the pressure-driven controller, which is characterized by its high stability, responsivity, and reproducibility, unlike syringe pump systems.
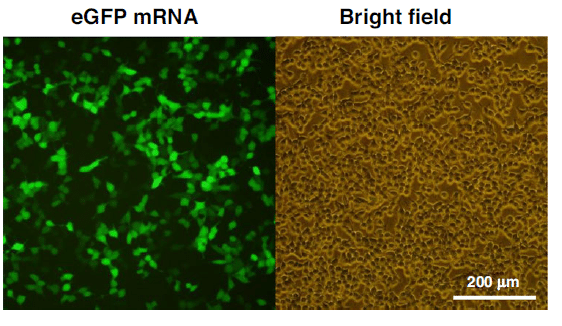
The transfection of the mRNA-loaded lipid nanoparticles into HEK-293T cells showed that eGFP was successfully expressed in the cytoplasm, with bright green fluorescence visble in positive cells under the fluorescence microscope.
These results indicated the practical application of the mRNA encapsulation module for possible mRNA vaccine preparation.
Conclusion
In this paper highlight, Hongjuan Wei et al., from the Bioinformatics center of AMMS (Beijing), succeeded in developing a universally integrated platform with a corresponding control system for the streamlined and on-demand preparation of mRNA products. Using our pressure-driven controllers (FlowEZ) and flow sensors (FlowUnit), an mRNA encapsulation module based on a staggered herringbone micromixing chipwas integrated into this platform. The mRNA-loaded lipid nanoparticles created with this module were stable and monodisperse.
Their transfection into cells was successfully demonstrated. This approach bringsforth new opportunities in terms of rapidly developing mRNA products in a streamlined process, which may help to facilitate access to mRNA technology. For example, mRNA vaccines developed for urgent epidemic issues and personalized treatments could be rapidly generated and tested at the early research and development stage.
Also, learn how to synthesize Liposome nanoparticles using Fluigent and Secoya’s Raydrop.
Related products
Related Content
Microfluidics for Pharmaceutical Applications
Read moreWebinar – Microfluidics in the manufacturing of sustainable nanomedicines
Read more
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Microfluidic Application Notes 1-10 microns PLGA microsphere production using the RayDrop Read more
-
Microfluidics Article Reviews Solid lipid nanoparticles for biologics and drug encapsulation Read more
-
Microfluidics Article Reviews Pressure-driven flow controllers vs. Syringe pumps: A flow precision evaluation for optical blood imaging. Read more
-
Microfluidics Article Reviews Microfluidic technology for engineered nanoparticles in nanomedicine Read more
-
Expert Reviews: Basics of Microfluidics Microfluidics for vaccine development Read more
-
Microfluidic Application Notes Liposome Nanoparticle Synthesis Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
References
- (1) Kose, N.; Fox, J. M.; Sapparapu, G.; Bombardi, R.; Tennekoon, R. N.; De Silva, A. D.; Elbashir, S. M.; Theisen, M. A.; Humphris-Narayanan, E.; Ciaramella, G.; Himansu, S.; Diamond, M. S.; Crowe, J. E. A Lipid-Encapsulated mRNA Encoding a Potently Neutralizing Human Monoclonal Antibody Protects against Chikungunya Infection. Sci. Immunol. 2019, 4 (35), eaaw6647. https://doi.org/10.1126/sciimmunol.aaw6647.
- (2) Alberer, M. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390, 1511–1520 (2017).
- (3) European Council Council of the European Union, https://www.consilium.europa.eu/en/infographics/covid-19-mrna-vaccine/