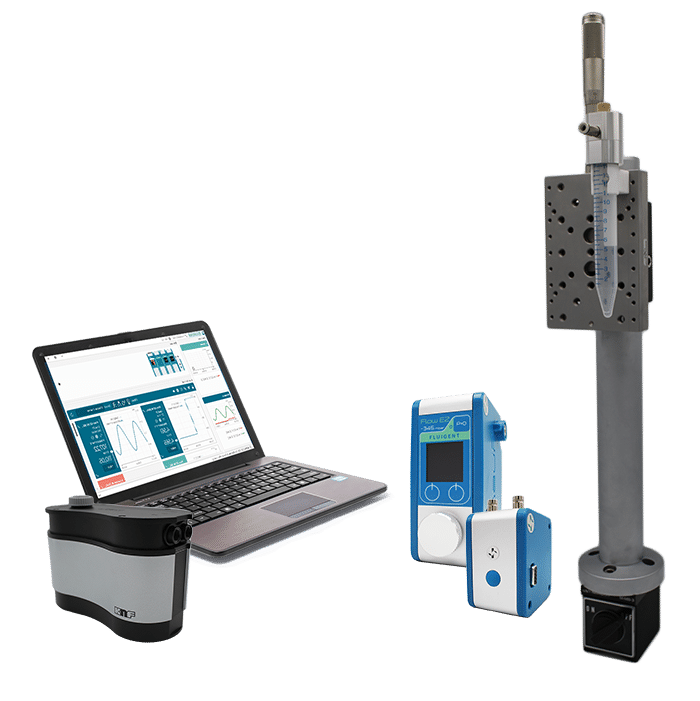
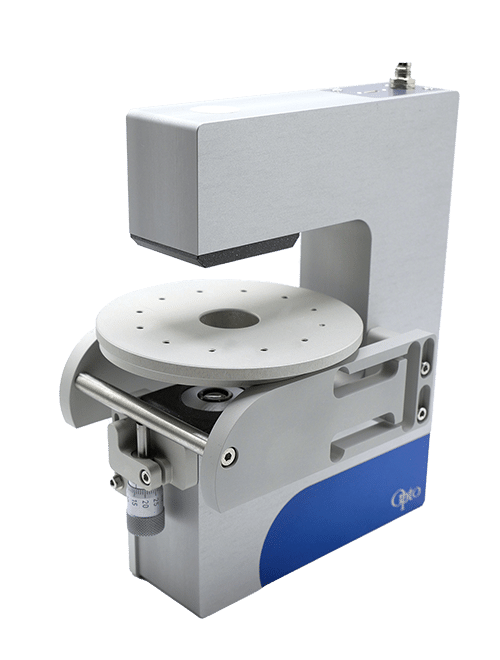
Micropipette aspiration of cells and tissues
Micropipette aspiration is a powerful non-invasive technique to evaluate how biomechanical properties of single cells or tissue govern cell shape, cell response to mechanic stimuli, transition from nontumorigenic to tumorigenic state or morphogenesis.
The Fluigent MFCS™-EZ and Flow EZ™ pressure controllers are particularly suited for this method since it requires applying forces ranging from 10pN to 1nN accurately.
How do we measure the mechanical properties of cells
What are the mechanical properties of cells?
The mechanical properties of the cell nucleus are increasingly recognized as critical in many biological processes. The deformability of the nucleus determines the ability of immune and cancer cells to migrate through tissues and across endothelial cell layers. The changes to the mechanical properties of the nucleus can also serve as novel biomarkers in processes such as cancer progression and stem cell differentiation [5].
Current techniques employed
However, current techniques to measure the viscoelastic nuclear mechanical properties are often time-consuming, limited to probing one cell at a time or require highly specialized equipment. Furthermore, many current assays do not measure time-dependent properties, which are characteristic of viscoelastic materials [5, 6].
Micropipette Aspiration relies on the suction/aspiration of a cell through a micropipette by the application of precise and sensitive negative pressure from a pressure based-controller. The cell is first immobilized on the tip of a micropipette and suction is applied to draw the cell inside the tube.
Why using the micropipette asipiration technique?
To quantify the distance traveled by the pipetted portion of a cell through the micropipette tube, the cell’s position can be tracked using a microscope. This behavior is predicted by a lumped parameter model which represents the cell as a combination of springs and dashpots (thereby modeling stiffness and viscosity, respectively) [5].
The deformation displayed by cells undergoing micropipette aspiration shows a linearly elastic response as a result of its stiffness, and a creep response due to its viscous behavior. This response is unique to different types of cells; for example, white blood cells measure lower stiffness than chondrocytes. This difference in cell stiffness affects the way in which cells interact with one another and the way they are affected by their immediate environment. pipette microaspiration is a versatile technique that allows stiffness to be quantified across many different cell types.
Being able to quantify the mechanical properties of cells can be a useful tool in investigating and diagnosing different pathologies. From handling delicate individual cells, along with the small forces required to manipulate them, and their small size, it is not feasible to use traditional methods of measuring material properties.
Benefits of pipette microaspiration
- Non-invasive: Micropipette suction allows repetitive measurements on the same sample. Variations in cell tension of individual cells within a tissue can be monitored over time. This method is a powerful tool to trace the spatio temporal map of tensions inducing morphogenesis (Maitre et al, Nat Cell Biol, 2015).
- Economical, easy to use and build: Micropipette aspiration with Fluigent is compact, can fit any microscope and is piloted by an intuitive software. Competitive technologies like AFM, cytoindenter and optical tweezers are expensive, necessitate specific training and may require a dedicated microscope.
- Time saving: Thanks to Fluigent’s product responsiveness, a given pressure is instantaneously applied on the cell’s surface (ms range). Cell surface tension can be measured in 3 to 5 minutes (Maitre et al, Nat Cell Biol, 2015)
- No inter-operator variability: Besides requiring highly skilled people, manual aspiration causes considerable inter-operator variability as the applied pressure cannot be exactly quantified. In contrast, Fluigent pressure regulators always deliver the ordered pressure with 0.1% precision.
- High sensitivity and resolution: Fluigent instruments are the only products in the market delivering small pressure increments (0.007mbar) at low pressure (0.1-10mbar). They allow investigating subcellular dynamics like cytoskeleton structural and organizational modifications that are not accessible with confocal microscopy.
Micropipette aspiration technique application
Micropipette suction remains one of the gold standards and most commonly used tools to study nuclear mechanics and provide important information on the viscoelastic behavior of the nucleus over different time scales.
Pipette microaspiration has been used to study a wide variety of phenomena, including the mechanical properties of the nucleus, the exclusion of nucleoplasm from chromatin, and chromatin stretching [5].
Image courtesy of Jean-Léon Maitre (Institut Curie, France)
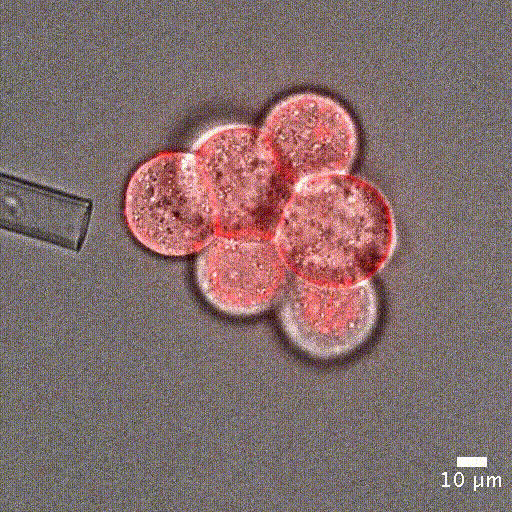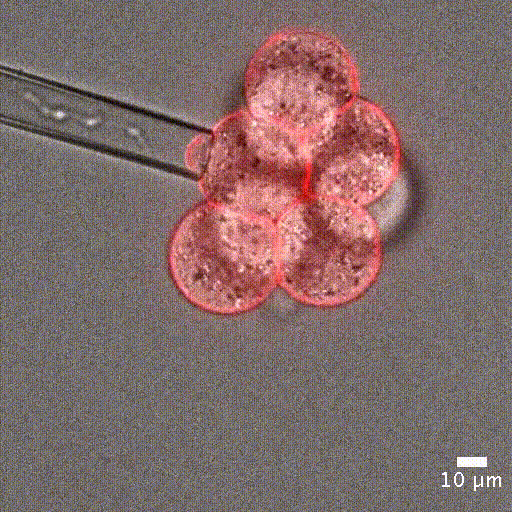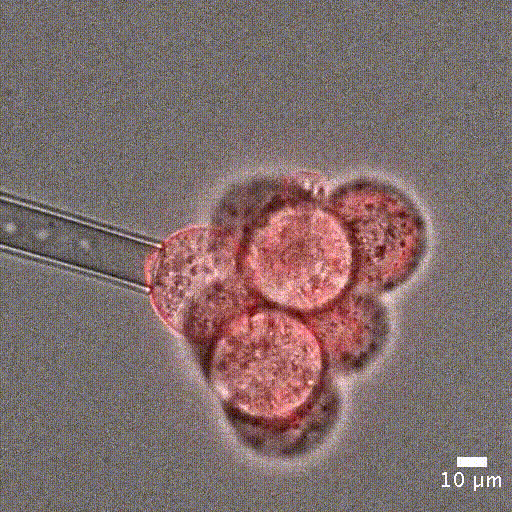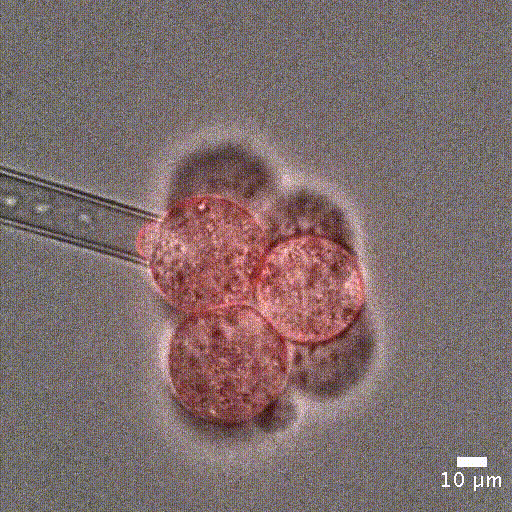
Cell mechanical properties measurement: Many biological processes are characterized by changes in cell stiffness: cells entering mitosis [1], tumor cells transitioning to premalignant stage [2], red blood cells infected with malaria [3].These mechanical changes occur at cell scale and require precise measurement to accurately quantify cell stiffness.
Dual pipette aspiration assay, the duplicated version of cell aspiration set up, is another handy tool to evaluate the relative contribution of cell-cell tension versus cell-medium tension at cell-cell interface by separating contacting cells (Maitre et al Sciences 2012).
Single cell manipulation: Micropipette aspiration allows spatial positioning of single cells or clusters of cells. Single-cell positioning is necessary for single-cell analysis or clonal cell line development.
Tension heterogeneity within tissue: Evaluating cell tension at the single cell level allows mapping the spatial map of tensions of a tissue. It is particularly performant to investigate the forces driving tissue morphogenesis or embryogenesis (Maitre et al, 2016, Nature).
In-vitro diagnostic: Measuring stiffness with cellular resolution can be a powerful tool to detect abnormal behavior that is not accessible nor perceptible under a microscope. As an example, mechanical properties can predict the viability of embryos already within hours after fertilization although viable and non-viable embryos are morphologically indistinguishable at this stage [4].
Micropipette aspiration package
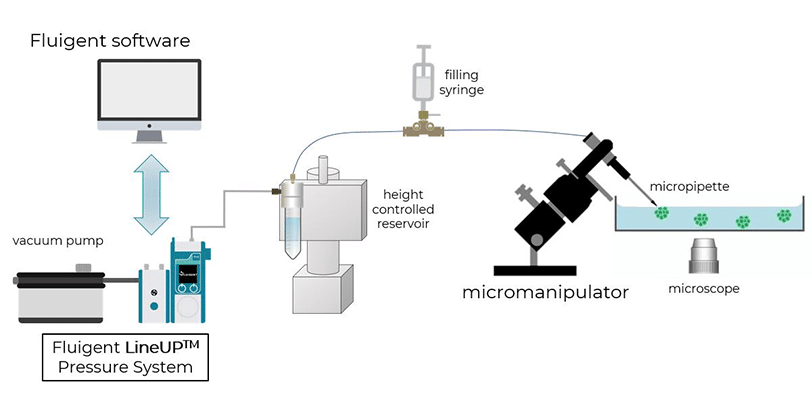
Expertises & Resources
-
Microfluidics White Papers An exploration of Microfluidic technology and fluid handling Read more
-
Microfluidic Application Notes Assess Cell Proliferation Using Pressure as a Tool Read more
-
Expert Reviews: Basics of Microfluidics High Throughput Single Cell Analysis Read more
-
Expert Reviews: Basics of Microfluidics Pump Responsiveness in microfluidics Read more
-
Expert Reviews: Basics of Microfluidics Flow Control Technologies: Comparison between peristaltic, syringe and pressure pumps for microfluidic applications Read more
References
[1] Théry M, Bornens M, Get round and stiff. 2008, HFSP J, 2(2):65-71.
[2] Tavares S et al, actin stress fiber organization promotes cell stiffening and proliferation of pre-invasive breast cancer cells. 2017, Nat Commun. 8:15237.
[3] Guo Q et al, Microfluidic biomechanical assay for red blood cells parasitized by Plasmodium falciparum. 2012, Lab Chip; 12(6):1143-50.
[4] Yanez LZ et al, human oocyte developmental potential is predicted by mechanical properties within hours after fertilization, 2016, Nat Commun. 7:10809
[5] Davidson, P.M. et al. (2019) “High-throughput microfluidic micropipette aspiration device to probe time-scale dependent nuclear mechanics in intact cells,” Lab on a Chip, 19(21), pp. 3652–3663. Available at: https://doi.org/10.1039/c9lc00444k.
[6] González-Bermúdez, B., Guinea, G.V. and Plaza, G.R. (2019) “Advances in micropipette aspiration: Applications in cell biomechanics, models, and extended studies,” Biophysical Journal, 116(4), pp. 587–594. Available at: https://doi.org/10.1016/j.bpj.2019.01.004.
Selected publications from our customers
Guevorkian K,Maître JL.Micropipette aspiration: A unique tool for exploring cell and tissue mechanics in vivo.MethodsCellBiol. 2017;139:187-201
Maître JL et al, Asymmetric division of contractile domains couples cellpositioning and fate specification, Nature. 2016 Aug 18;536(7616):344-34
Biro M, Maître JL, Dual pipette aspiration: a unique tool for studying intercellular adhesion.MethodsCellBiol. 2015;125:255-67
Porazinski S et al, YAP is essential for tissue tension to ensure vertebrate 3D body shape.Nature. 2015 May 14;521(7551):217-221
Maître JL et al, Pulsatile cell-autonomouscontractility drives compaction in the mouse embryo. Nat Cel lBiol. 2015 Jul;17(7):849-55
Maître JL et al, Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells.Science. 2012;338(6104):253-6