Key considerations for fluidic system integration
Scientific progress and workflow automation have driven the transition from manual processing to automated solutions in laboratories. Automation is essential for in vitro diagnostics (IVD), molecular analysis, flow cytometry, next-generation sequencing (NGS) or cell biology systems. The right choice of fluid management methods can often determine the success of a project, as well as the overall time and cost of manufacturing. Here’s what you need to consider and be aware of as you plan for fluidic system integration.
Fluid considerations when integrating fluidics into your system
How will pulsatile flow affect system performance?
Droplet-based applications: Droplet digital PCR and cell encapsulation for single-cell analysis usually make use of droplets generated by microfluidic technology. These applications require liquid handling systems that produce flow with very low to no pulsation to obtain homogeneous droplets, which is vital for the success of these applications.
Flow cytometry and surface plasmon resonance (SPR) are based on real-time analytical measurements of samples passing by a detection area. They require a fluid handling delivery system with a stable flow rate to deliver fluids through the fluidic channels.
Live cell imaging: In live-cell imaging applications with fluid perfusion, pulsatile flow leads to variable shear stress imposed on cells, and may impact cell viability. Liquid flow should be stable and controlled.
In some applications, a steady flow rate is a prerequisite for reliable operation after fluidic system integration:
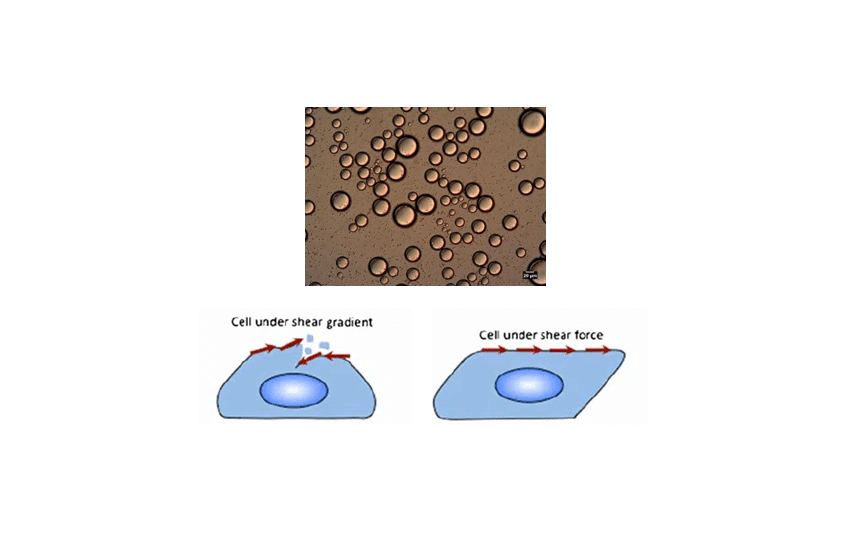
For these applications, pressure-based fluid delivery can deliver flow rates in the microliter- or nanoliter-per-minute range with a stable flow rate (high precision and accuracy) and no pulsation.
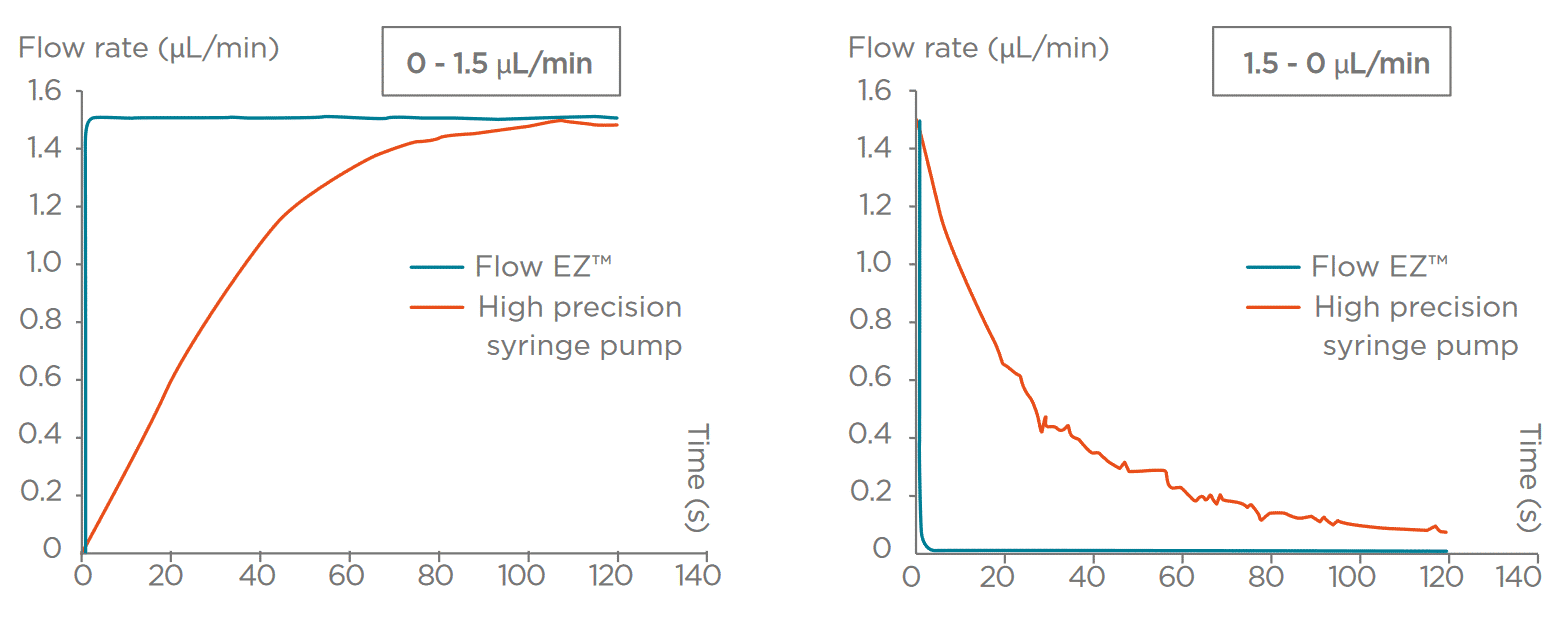
Do you require fast settling times?
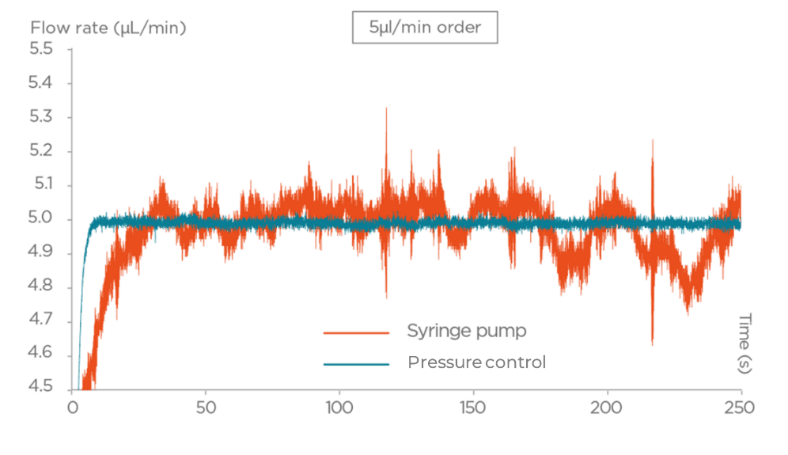
Some applications call for alternating between multiple different flow rates in a short period of time. This is common for flow cytometry, cell sorting, or fluorescent activated cell sorting (FACS). In the latter, 3 flow rate modes are usually available: slow, medium, and fast, and one can switch from one to another.
For fluidic system integration in applications like these, consider using a fluid delivery solution that allows for rapid response time (a few seconds). Pressure-based flow control has the advantage of having a quasi-immediate response. A step of several bars can be made within milliseconds, and the liquid flow rate reaction is equally fast, allowing the system to reach typical microfluidic flow rates in less than one second. This cannot be achieved by most motors used in syringe pumps, as revealed by the graph below.
Is sterility required?
Many biological applications require a sterile environment, including cell culture under perfusion, immunostaining, organoid culture, organ on a chip, drug discovery, single-cell analysis and cell cytometry, among others. When using fluids such as culture media, PBS, buffers, blood or plasma, every component in the fluidic path should be disposable or sterilizable.
For these applications, pressure-based liquid delivery systems that do not contact fluids or have sterilizable components are ideal. As for liquid handling without flow rate monitoring, using pressure allows the system to use disposable tubing only between the inlet reservoirs and the application. At the fluidic system integration stage, however, you may want to plan for the ability to monitor or control the flow rate. The question of sterility arises when choosing the flow rate metering solution. Most flow sensors are too costly to be considered as disposables, yet are in contact with the fluids. A non-intrusive flow sensing technology is a particularly interesting way to tackle this issue.
Do you need to handle multiple fluid streams?
Multiple streams are useful for operations that perform several tests simultaneously, such as drug screening where different drug candidates are tested, or personalized medicine applications that make use of tumor biopsy studies. It is also sometimes convenient and cost-effective to separate a fluid originating from one liquid delivery system into two distinct paths.
It’s important to choose the right liquid delivery system when designing for multiplexing. Depending on your choice, an increased number of systems may be highly expensive, and fluidic system integration can be cumbersome. Using fluidic valves or quake valves can also be an interesting alternative, as they allow for fluid management with a reduced number of fluid delivery systems.
What are the volumes to be dispensed?
The volumes to be injected are highly application-dependent. For instance, in cell biology applications such as cellular imaging or dynamic cell culture, fluids may be injected over a period of several days. In these applications, high reservoir volumes (up to 1 L) are required. Another option could be recirculating media or reagents with a peristaltic pump, a recirculation valve or a fully integrated solution. Conversely, drug screening applications may make use of expensive liquids, and only very small amounts of fluids should be used (< 100 µL).
Depending on the liquid delivery method used in the fluidic system, reservoir size can affect precision and accuracy. For example, when using syringe pumps, smaller syringes maximize accuracy for small volumes but require frequent refilling for larger volumes. Larger syringes will increase capacity, but will lose accuracy and become pulsatile at lower flow rates. Using a peristaltic pump for fluid recirculation is relatively uncomplicated.
Do you need to control or measure flow rate?
For most liquid delivery applications, the user wants to set a desired flow rate and have a solution to reach it via fluidic system integration through use of a feedback loop algorithm. Syringe or peristaltic pumps give easy access to the real time volumetric flow rate, but pressure controllers do not.
To overcome this, the user can add a flow sensor to the configuration that can either be connected to the pressure controller for flow rate control, or used for monitoring only. There are several flow sensing technologies based on different approaches, each with their respective advantages and limitations. The best choice for a given application depends on your requirements with regard to response time, chemical compatibility, flow rate range, cost, footprint and other parameters. For microfluidic applications in particular, one must also be cautious about channel and fluid interactions, the formation of bubbles, and multi-phase flows, although these can be neglected at a larger scale.
Important considerations when integrating fluidics into your system
Time to market?
In a fast-growing market sector, it’s crucial to minimize time-to-market and launch new technologies before the competition. To accelerate development, consider pre-built components or solutions, such as liquid handling components that can be integrated quickly into your system or prototype. Fluigent’s standard and customizable liquid handling OEM components offer flexibility and ease of integration into your device. Using a readymade solution avoids the hassle of dealing with multiple fluid handling issues and leaves you more time to focus on your core expertise and application.
If you need expertise in fluidic system integration and are looking for a company that can do it for you, choose one that’s reactive, communicates with its customers, and can respect short timelines to achieve your desired launch date. At Fluigent, we design and manufacture all of our products under one roof at our headquarters in the Paris region. Our R&D and production teams work closely with each other and with our customers to ensure maximum satisfaction.
Do you have all the resources needed for in-house development?
Handling fluidic system integration by yourself is an option when developing an automated liquid handling system. The conception, design and development of the system will require engineering expertise in the mechanical, electrical, software and manufacturing domains.
When developing a system internally, you need to be sure you have the experience and engineering knowledge to translate your proof-of-concept device into a reliable and efficient automated system. The entire product development process is at risk if you are missing any of the required resources.
Related expertises & ressources
- Microfluidics White Papers
An exploration of Microfluidic technology and fluid handling
Read more - Expert Reviews: Basics of Microfluidics
What is the history of microfluidics?
Read more - Expert Reviews: Basics of Microfluidics
Microfluidics overview: History and Definition
Read more - Array
Microfluidic Flow Control Technologies: Strengths and Weaknesses
Read more 
5 reasons to choose OEM pressure controllers over OEM syringe pumps for microfluidic applications
Read more
Microfluidics in Food Industry: Food Testing & Agriculture
Read more
Microfluidics in Water analysis
Read more
Compact All-In-One Microfluidic Micropump
Read more
Related products
-
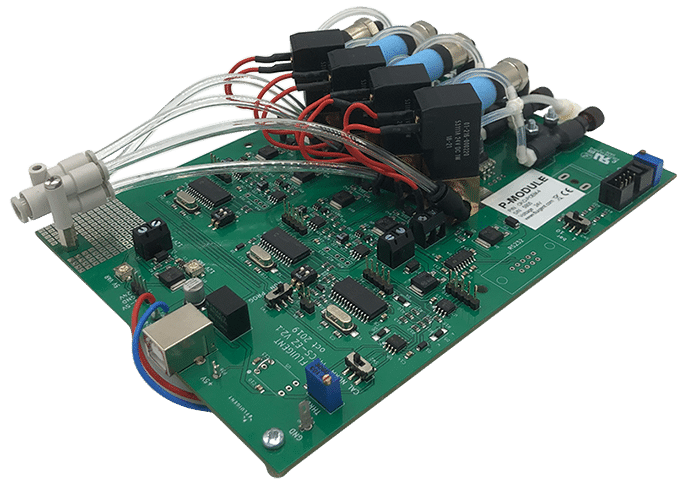 Microfluidic Flow Management Unit Read
Microfluidic Flow Management Unit Read -
 (Patent pending) compact all-in-one pressure supply & pressure/flow control Read
(Patent pending) compact all-in-one pressure supply & pressure/flow control Read -
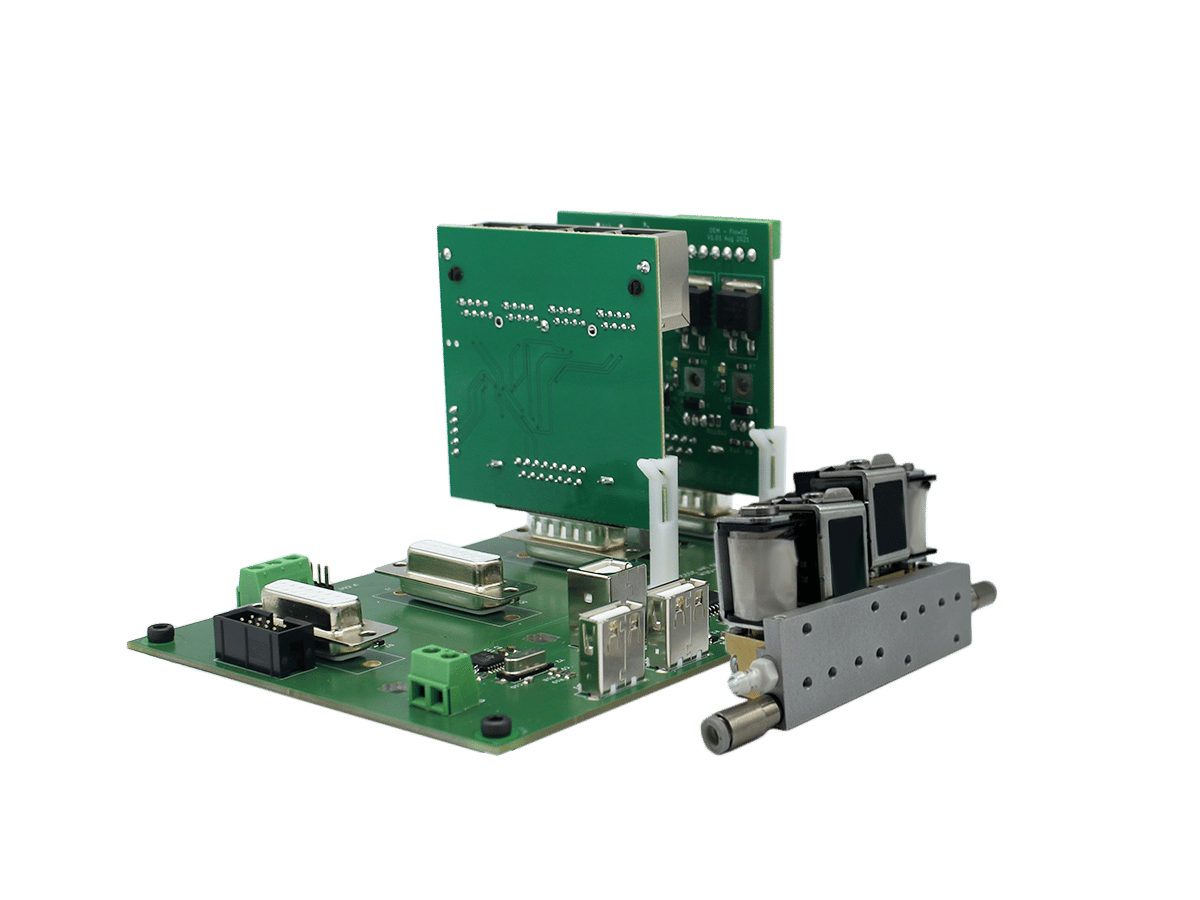 Modular OEM Microfluidic Flow Controller Read
Modular OEM Microfluidic Flow Controller Read -
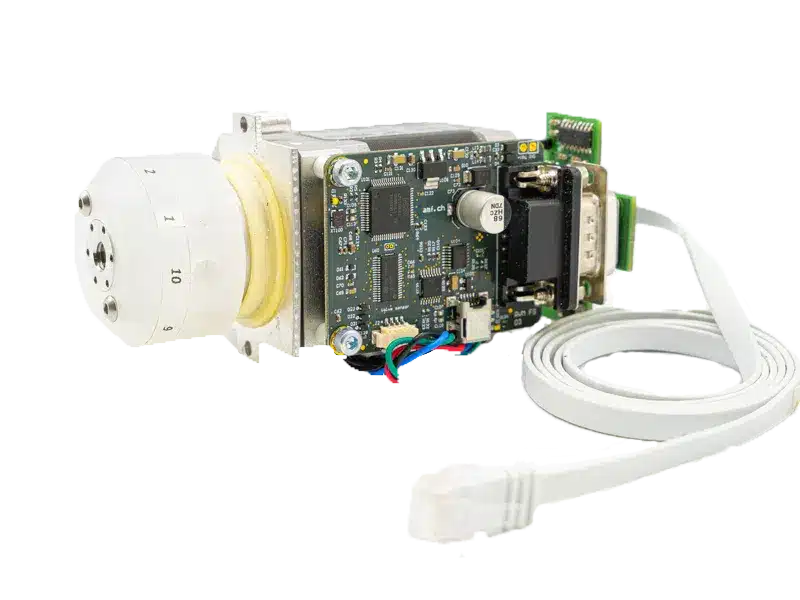 Rotary multi-port microfluidic valve for industry Read
Rotary multi-port microfluidic valve for industry Read -
 Sample injection and recirculation microfluidic valve for industry Read
Sample injection and recirculation microfluidic valve for industry Read -
 Lab Integration Software Read
Lab Integration Software Read -
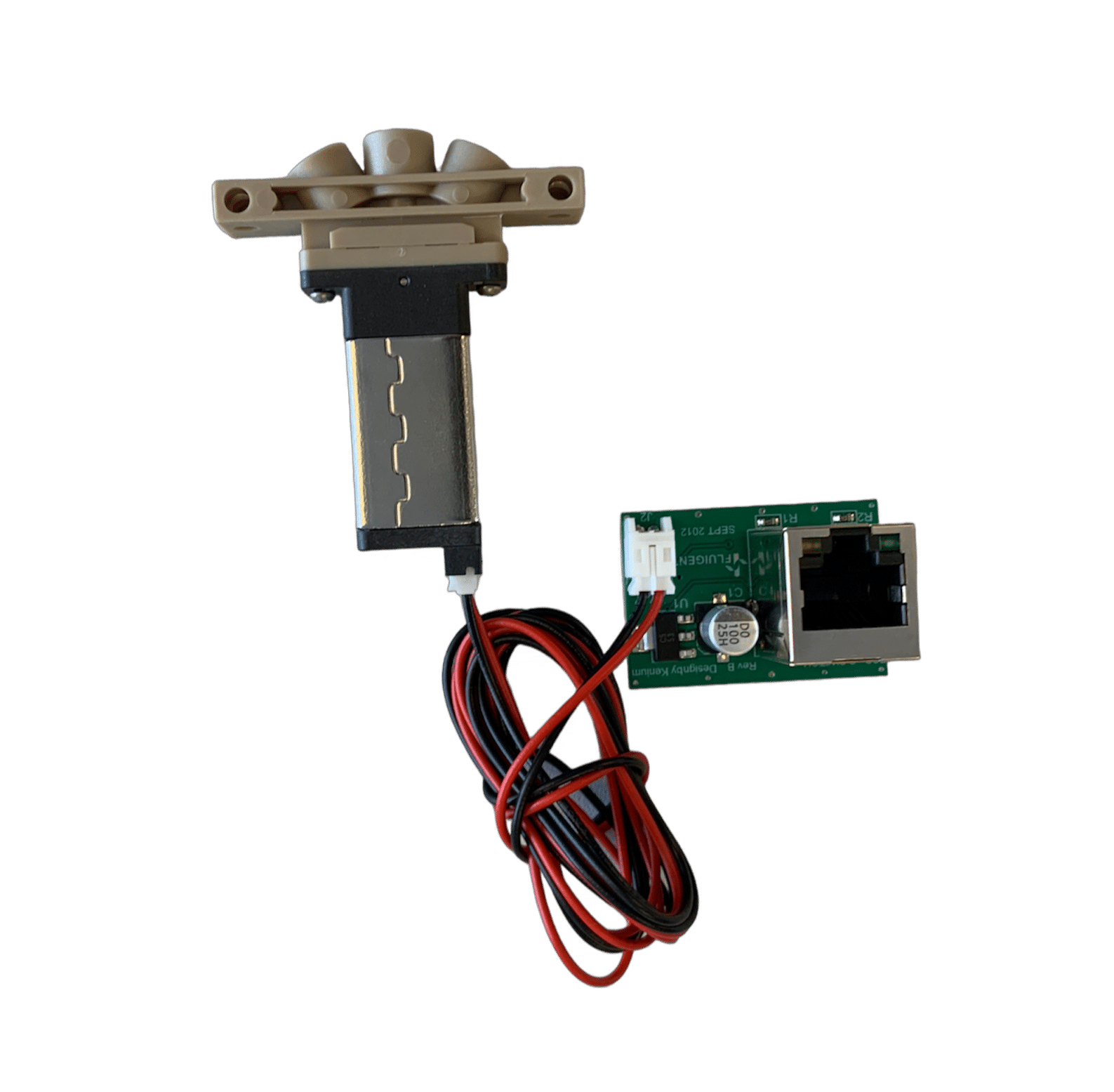 3-port/2-way bidirectional valve for industry Read
3-port/2-way bidirectional valve for industry Read -
 Microfluidic OEM Pressure Controller Read
Microfluidic OEM Pressure Controller Read -
 OEM Microfluidic Pressure Source Read
OEM Microfluidic Pressure Source Read -
 Omi, an Automated Organ-On-A-Chip Platform Read
Omi, an Automated Organ-On-A-Chip Platform Read