10 Tips for Reliable Droplet Generation
Droplet-based microfluidics requires precise liquid control for applications in drug discovery, diagnostics, and material synthesis. This guide provides 10 best practices for reliable droplet generation to improve droplet or particle stability and monodispersity in routine use.
Introduction: Mastering Droplet-Based Microfluidics
Droplet-based microfluidics is a versatile microfluidic application area focused on creating and manipulating small, discrete volumes of liquids using immiscible fluid flows.1 First explored nearly two decades ago by Thorsen et al., and further developed by H. Stone, D. Weitz, P. Tabeling this technology has enabled the consistent formation of droplets with uniform size and shape, offering significant advantages over traditional methods (spray drying, centrifugal droplet generation, ultrasonic atomization or bulk mixing).2
The ability to generate large numbers of uniform droplets and the ability to encapsulate cells, biologicals, and other materials in them, has made droplet microfluidics essential in fields such as drug discovery, enzyme kinetics, single-cell sequencing, and combinatorial synthesis. It also supports lab-on-a-chip technologies, advancing personalized medicine, diagnostics, cell culture, tissue engineering, and drug delivery. 3–7
Despite its potential, issues surrounding droplet stability, monodispersity, and maintaining sample integrity during manipulation remain. This guide provides 10 essential tips to help you generate droplets efficiently, minimize troubleshooting, and improve droplet formation.
1- Choose the Right Microfluidic Chip Design for Droplet Generation
The design of the microfluidic chip is fundamental for controlling droplet size and simple droplet generation. Here are some common designs (Figure 1):8
- Coaxial Geometry: The continuous phase surrounds the dispersed phase in a 3D configuration, providing uniform droplets and better control over droplet formation. However, it requires complex fabrication.9
- Co-flow Geometry: Dispersed phase flows inside the inner capillary, offering control over droplet size with simpler fabrication than coaxial.10
- Flow-focusing Geometry: Opposing flows pinch off droplets at a narrow constriction, offering stable droplet formation but with more complex fabrication.11,12
- Cross-flow Geometry: Phases meet at a T-junction, ideal for low flow rates and uniform droplets, but less precise than other designs.2
- Step-emulsification Geometry: Droplets form when the dispersed phase flows through a sudden increase in channel size, ideal for high-throughput, monodisperse droplets.13
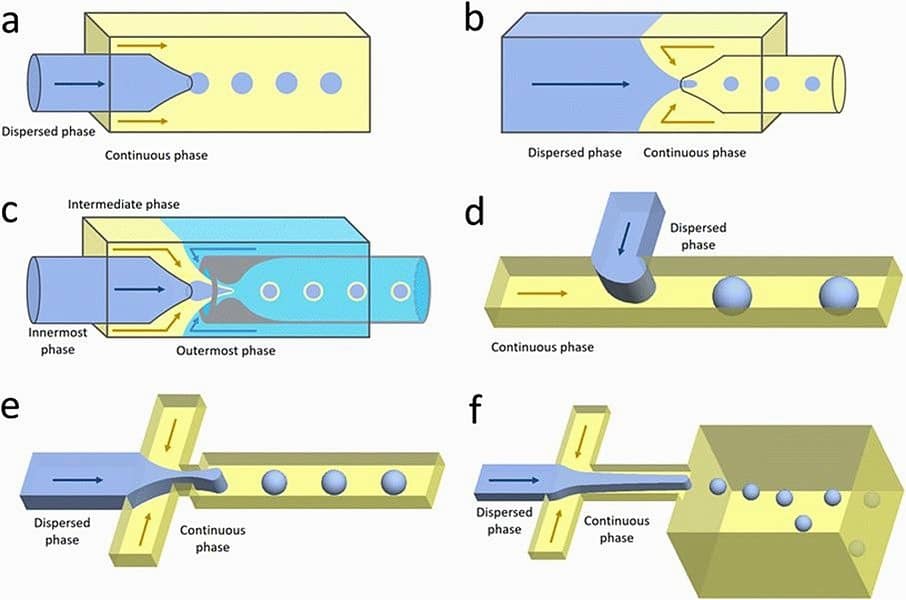
Selecting the right geometry depends on the required droplet uniformity, throughput, and fabrication complexity. While simpler designs like co-flow and cross-flow are easier to manufacture, more complex designs like coaxial and step-emulsification provide better control and precision.

Discover Secoya Technologies’ RayDrop: combining co-flow and flow-focusing with an exchangeable nozzle for practical transition between single and double emulsion.
Discover how to choose the right microfluidic chip and explore the microfabrication process in detail.
2- Select the Right Material for the Microfluidic Chip
The choice of material impacts the performance, fabrication complexity, and scalability of microfluidic devices. The main material classes can be classified into three types:14
- Inorganic Materials (Glass & Silicon): Offer excellent chemical resistance, mechanical rigidity, and high optical clarity. However, they can be expensive and challenging to fabricate, requiring photolithography and wet-etching techniques. Despite the high cost, glass devices can be washed and reused.9
- Elastomers (PDMS): A low-cost, easy-to-fabricate alternative with excellent flexibility. PDMS is typically patterned using soft lithography and can be bonded to glass or other PDMS layers. However, it has poor compatibility with chemical solvents, limiting its use in some applications. Additionally, PDMS tends to absorb small hydrophobic molecules, which can interfere with biological or chemical assays by reducing analyte concentration or introducing unwanted contamination.15
- Thermoplastics (PMMA, PC, PS, PVC, COC): Enable large-scale manufacturing via injection molding or hot embossing. While ideal for high-throughput production, small-scale fabrication requires micromachining, which has lower resolution compared to lithography. Some thermoplastics and elastomers can also be 3D printed, though resolution varies.14,16
The best material depends on your application. Glass and silicon provide high precision but are costly, PDMS offers flexibility and ease of fabrication, while thermoplastics allow for mass production but have lower resolution for small-scale features.
Table 1: Inorganic materials, elastomers and thermoplastics characteristics14 (Adapted from Elvira, K.S. et al.; Lab. Chip 22, 859–875 (2022).
| Property | Inorganic Materials (Glass, Silicon) | Elastomers (PDMS) | Thermoplastics (PMMA, PTFE) |
|---|---|---|---|
| Chemical Compatibility | High | Moderate | Moderate to Good |
| Thermal Stability | High | Moderate | Variable |
| Surface Hydrophilicity | Hydrophilic | Typically Hydrophobic | Typically Hydrophobic |
| Physical Patterning | Laser Ablation, Micromachining, Chemical Etching | Casting, 3D Printing | Micromachining, Moulding, Laser Ablation, 3D Printing |
| Fabrication Time | High (due to complex processes) | Medium (depends on casting or 3D printing) | Medium (depends on process complexity) |
| Cost | High (due to specialized equipment and processes) | Medium (relatively affordable materials, but casting and 3D printing costs vary) | Medium (depending on material choice and process complexity) |
Learn more about selecting the right chip and the microfabrication process of microfluidic chips.
3- Control Wetting and Surface Treatment
The interaction between fluids and the channel surface controls which fluid becomes the continuous phase and which becomes the dispersed phase. With small channel sizes and high surface area to volume ratios, the channel/fluid interface has a dominant effect on fluid behavior. This can be managed through material selection or surface modification techniques:
- Material Selection: Choosing materials with appropriate surface properties ensures proper wetting (Table 1). For example, hydrophilic surfaces will preferentially wet aqueous fluids, promoting oil-in-water droplet formation. Hydrophobic surfaces are ideal for water-in-oil droplets. The contact angle is an important factor in determining droplet type; a critical contact angle dictates whether water-in-oil or oil-in-water droplets are formed.14,16–20
- Surface Modification: When the native surface properties are not suitable, surface modifications are needed. Techniques such as plasma treatment, oxidation, and silanization can adjust the surface properties. For instance, glass can be modified for both oil-in-water and water-in-oil droplets, while PDMS often requires surface treatment to maintain desired properties for long-term droplet formation.21–23
Proper material selection and surface treatments such as plasma treatment, oxidation, and silanization are essential for achieving stable and controlled droplet formation in microfluidic devices.
4- Use Surfactants to Stabilize Droplets
Surfactants, or emulsifiers, are amphiphilic molecules that stabilize the fluid-fluid interface and can temporarily modify the channel surface. By changing the surfactant, both water-in-oil and oil-in-water droplets can be generated in the same device without additional surface modifications.24
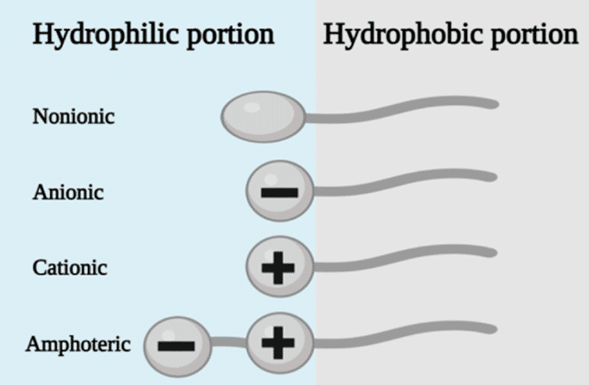
- Surfactant Function: Common surfactants include anionic surfactants like SDS and non-ionic surfactants such as Span 80, Tween 20, and PEG. These surfactants stabilize droplets by temporarily altering surface chemistry (Table 2).25
- Surfactant Placement: Surfactants can be added to the disperse or continuous phase. In the continuous phase, surfactants migrate to the channel/fluid interface, where they coat the surface. Devices are often primed by flowing the continuous phase before introducing the disperse phase.25,26
Surfactants stabilize droplets and temporarily modify channel surfaces, enabling precise control over droplet formation.
Tableau 2: Summary of surfactant types and their characteristics. 27,28
| Type | Characteristics | Examples |
|---|---|---|
| Anionic | The hydrophilic group carries a strong negative charge High irritation and acute toxicity potential | Sodium Lauryl Sulfate (SLS) Sodium Dodecyl Sulfate (SDS) |
| Cationic | The hydrophilic group carries a strong positive charge Commonly used for cosmetic products | Stearalkonium Benzalkonium Trimethyl ammoniums such as Cetyltrimethylammonium Bromide (CTAB) |
| Amphoteric | Present both negative and positive charges. Its final charge depends on the pH Milder and less irritating | Hydroxysultaines Coco Bentaine Lauryl Bentaine |
| Non anionic | The hydrophilic group has no charge Often used in drug delivery systems, biological testing, and food emulsification applications | PEGs Sorbitans Polysorbates Tweens and Spans |
5- Improve Stability with Pressure Controllers
Precise control of flow is crucial for reliable droplet generation in microfluidic experiments. The size and monodispersity of droplets depend directly on the flow precision, making accurate flow control essential for obtaining repeatable and reliable results. Choosing the right flow control method can significantly impact the quality of the droplets:
- Syringe Pumps: Based on mechanical action, syringe pumps introduce pulse errors and have limited flow control, leading to inconsistencies in droplet size and making it difficult to achieve repeatable reactor volumes.
- Pressure-Based Flow Controllers: These systems offer high-precision flow control, faster reaction times, and continuous flow monitoring, ensuring consistent droplet size and eliminating the pulse errors seen with syringe pumps.
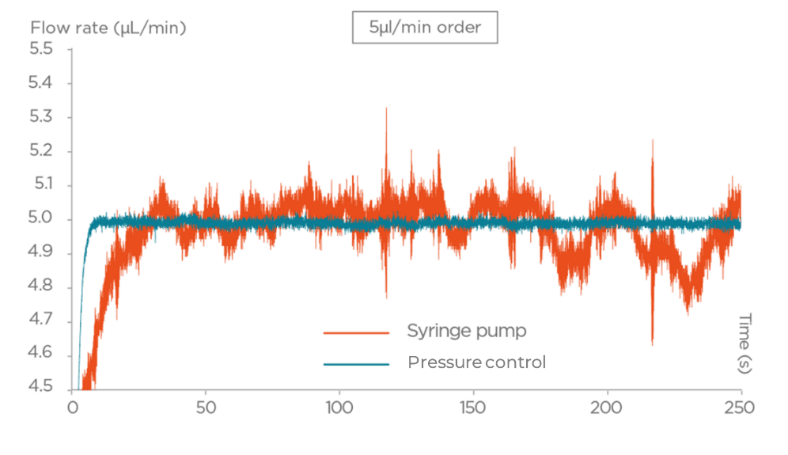
For more reliable and consistent droplet generation in microfluidic systems, pressure-based flow controllers are a superior alternative to syringe pumps. Their precise control and pulse-free operation ensure more reproducible experimental results.
Learn more about the effect of pressure-based controller for droplet formation compared to syringe pumps.
6- Optimize Flow Rate Ratios for Monodispersity
In microfluidic droplet generation, the balance of inertial and viscous forces influences droplet size and consistency. Key parameters, such as fluid phase properties and flow rates, directly impact the size, shape, and structure of droplets. The capillary number (Ca) is a crucial factor in this process, determining how flow rate adjustments affect droplet formation.29,30
- Phase Flow Rates and Capsule Size: The flow rates of the dispersed (Qd) and continuous (Qc) phases are directly related to capsule size. Increasing Qc while keeping Qd constant reduces capsule size. The Qd/Qc ratio is essential for optimizing droplet size and achieving monodispersity.
- Double Emulsion and Shell Thickness Control: Adjusting flow rates in double emulsion systems allows for control over both capsule size and shell thickness. For instance, Increasing Qd (shell phase flow rate in the case of double emulsion) thickens the shell without altering capsule size.
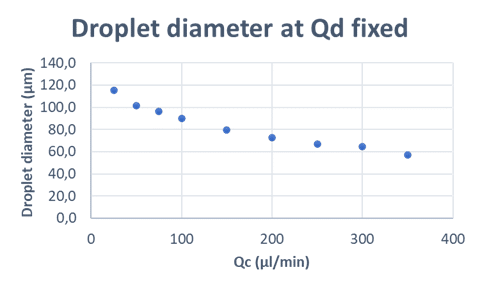
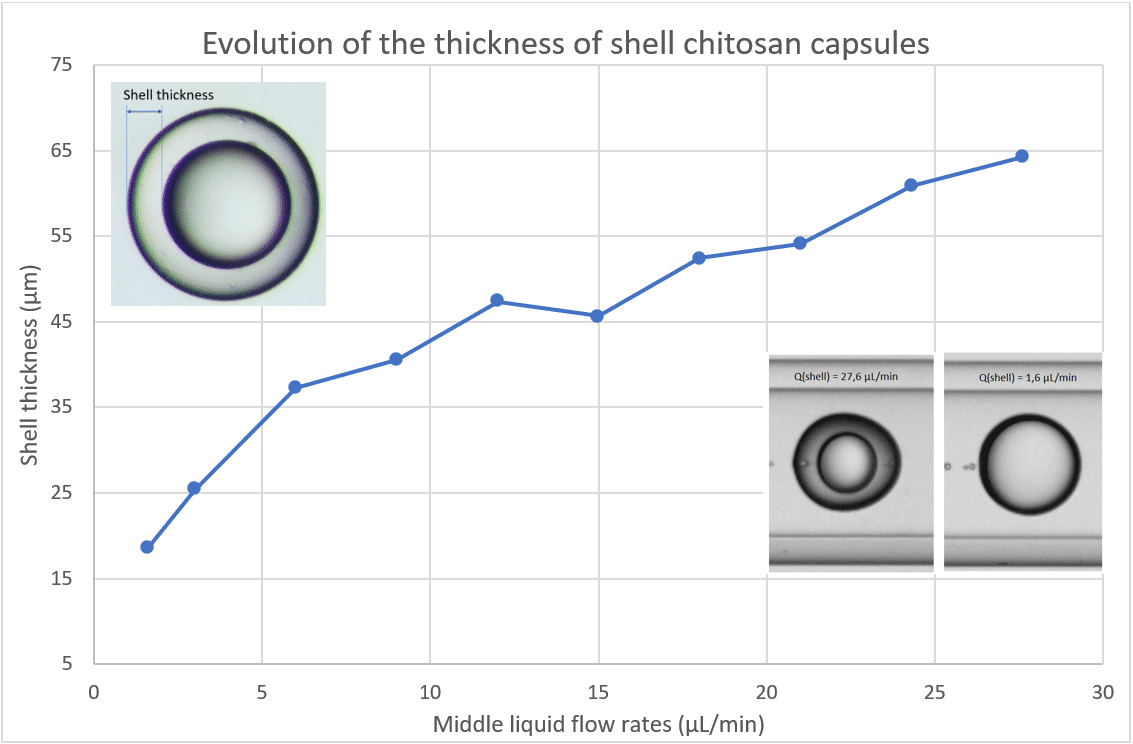
Optimizing the flow rate ratios of dispersed and continuous phases is key to achieving consistent droplet size and monodispersity in microfluidic systems. Fine-tuning these ratios enhances the reproducibility and precision of droplet generation.
For more information regarding the influence of flow rates on the droplet size, check out our application note about chitosan microcapsules.
7- Monitor and Troubleshoot Issues in Real Time
Real-time monitoring is essential to identify and resolve issues as they arise, ensuring consistent and accurate results. For instance, by utilizing advanced tools like flow rate real time control software and high-speed cameras, you can continuously track key parameters and make adjustments instantly.
- High-Speed Cameras: Microscope-integrated high-speed cameras offer real-time visualization of droplet formation. These cameras allow for close inspection of droplet size, uniformity, and any potential issues such as clogging or instability.
- Oxygen Fluigent Software: This software enables precise control and monitoring of flow rates in real time, allowing for quick adjustments to maintain optimal droplet formation conditions. It provides valuable data on pressure, flow rate, and system performance.
Real-time monitoring with tools is critical for ensuring smooth droplet generation in microfluidic systems. By detecting and troubleshooting issues immediately, you can maintain consistent results and optimize performance throughout the experiment.
8- Avoid Bubble Generation for a Stable System
Air bubbles in microfluidic systems can disrupt flow stability, affect response time, and even cause clogging, leading to unreliable results. These bubbles can originate from dissolved gases, leaks, or the permeability of materials like PDMS, which allow air diffusion through device walls. Preventing bubble formation is crucial for maintaining smooth and reproducible experiments.31
- Understanding Bubble Formation: Bubbles can arise from dissolved gases in liquids, porous materials, or improper system filling. Materials like PDMS are gas-permeable, allowing air to gradually accumulate in microchannels.
- Prevention Strategies: Degassing solutions before use, selecting low-permeability materials, and using hydrophilic surface treatments help minimize bubble formation. Implementing bubble traps or inline degassers further ensures a bubble-free system.
Preventing bubbles in microfluidic setups is essential for maintaining stable and reliable flow. By degassing solutions, choosing appropriate materials, and incorporating bubble traps, researchers can significantly reduce disruptions and enhance the accuracy of their experiments.
Check our expertise page regarding bubble issues in microfluidics and how to avoid them.
9- Optimize Your Microfluidic System by Integrating Complementary Tools
Enhancing your microfluidic setup with practical tools improves efficiency, precision, and ease of handling. Integrating fluidic control valves for precise sample injections, adapting the system based on the crosslinking method, and utilizing UV crosslinking modules ensures better encapsulation and stability in droplet-microfluidics.
- Easy Fluid Handling with Valves: Tools such as the Fluigent L-Switch enable precise injection of small sample volumes, which is particularly beneficial when working with rare or sensitive cells, such as stem cells or patient-derived samples. This allows you to efficiently manage limited volumes, reduce waste, and improve experimental control.
- Adapting the Setup to the Crosslinking Method: The shell of microcapsules plays a crucial role in protection and function. Depending on the encapsulation process, the system should be adjusted to match the crosslinking method:
- Physical Curing: Natural macromolecules such as gelatin, alginate, and chitosan solidify under changes in pH, temperature, or ionic strength.29,30,32
- UV Crosslinking: Synthetic polymers like polyacrylamide, polystyrene, and poly(ethylene glycol) diacrylate (PEGDA) are crosslinked using UV light or heat. PEGDA is particularly useful due to its tunable properties for biomedical applications.33–35
For instance, you can consider a UV crosslinking module which allows for exposure to a UV source to enable polymer crosslinking. The adjustable tubing inclination simplifies collection while preventing coalescence for superior encapsulation results.
Integrating fluidic control valves, adapting the setup for different crosslinking techniques, and using a UV crosslinking module enhance precision, reproducibility, and efficiency in microfluidic experiments. These tools ensure better sample handling, stable encapsulation, and high-quality microcapsules.
Discover our UV-crosslinked microcapsule production platform
10- Keep a Clean Setup and Use Proper Cleaning Protocols
A clean microfluidic setup is essential for preventing clogs, contamination, and maintaining consistent fluid flow during experiments. Proper filtration, cleaning protocols, and maintenance techniques are key to ensuring smooth operation and reproducible results.
- Pre-filter Solutions and Use Inline Filters: Always filter solutions before introducing them to the microfluidic setup to remove particulates that could obstruct the channels. Additionally, using inline filters in the microfluidic circuit allows for real-time filtration, ensuring that contaminants are captured as they enter the system and preventing blockages from affecting fluid flow during the experiment.
- Follow Cleaning Protocols and Resolve Clogs Efficiently: Tailor your cleaning protocol to the chemicals in use. Regular cleaning prevents chemical buildup and contamination. If the system becomes clogged, especially if it’s made of glass, perform a backflush by reversing the flow direction. This action will help clear any blockages and restore the function of the microfluidic channels without damaging the setup.
As an example of a microfluidic cleaning accessory, explore our method for cleaning the flow unit in this protocol.
Conclusion
Precision is key to achieving reliable, reproducible and easy droplet generation in your microfluidic experiments. By optimizing flow rates, preventing contamination, and integrating the right tools, you can enhance your results and streamline your workflow. Don’t hesitate to experiment and fine-tune your setup to achieve the best performance.
For even better control, see Fluigent’s solutions for precise flow rate management and Secoya Technologies’ RayDrop for advanced droplet generation technology. Our solutions can help take your experiments to the next level.
Related Products
Related Resources
-
Microfluidics case studies Microfluidic Transistor for Precise Fluid Control Read more
-
Expert Reviews: Basics of Microfluidics Giant Unilamellar Vesicles (GUVs) Production using Microfluidics Read more
-
Expertise Addressing Air Bubble Issues in Microfluidic Systems Read more
-
Microfluidic Application Notes A quick and efficient double encapsulation method for FACS-based droplet sorting Read more
-
Expert Reviews: Basics of Microfluidics Choosing the Right Microfluidic Pressure Range Read more
-
Microfluidic Application Notes Microbubble formation using the RayDrop Read more
-
Expert Reviews: Basics of Microfluidics How to choose a microfluidic chip Read more
-
Microfluidic Application Notes Microfluidic Chitosan Microcapsules Production Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Droplet Production Method Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Flow Control: Comparison between peristaltic, syringe and pressure pumps for microfluidic applications Read more
-
Expert Reviews: Basics of Microfluidics Microfabrication of Microfluidic Chips: Materials and Methods Read more
References:
1. Shi, N., Mohibullah, M. & Easley, C. J. Active Flow Control and Dynamic Analysis in Droplet Microfluidics. Annu. Rev. Anal. Chem. 14, 133–153 (2021).
2. Thorsen, T., Roberts, R. W., Arnold, F. H. & Quake, S. R. Dynamic Pattern Formation in a Vesicle-Generating Microfluidic Device. Phys. Rev. Lett. 86, 4163–4166 (2001).
3. Chiu, D. T. & Lorenz, R. M. Chemistry and Biology in Femtoliter and Picoliter Volume Droplets. Acc. Chem. Res. 42, 649–658 (2009).
4. Kim, S. C., Clark, I. C., Shahi, P. & Abate, A. R. Single-Cell RT-PCR in Microfluidic Droplets with Integrated Chemical Lysis. Anal. Chem. 90, 1273–1279 (2018).
5. Price, A. K., MacConnell, A. B. & Paegel, B. M. h ν SABR: Photochemical Dose–Response Bead Screening in Droplets. Anal. Chem. 88, 2904–2911 (2016).
6. Teh, S.-Y., Lin, R., Hung, L.-H. & Lee, A. P. Droplet microfluidics. Lab. Chip 8, 198 (2008).
7. Sjostrom, S. L., Joensson, H. N. & Svahn, H. A. Multiplex analysis of enzyme kinetics and inhibition by droplet microfluidics using picoinjectors. Lab. Chip 13, 1754 (2013).
8. Nan, L., Zhang, H., Weitz, D. A. & Shum, H. C. Development and future of droplet microfluidics. Lab. Chip 24, 1135–1153 (2024).
9. Utada, A. S. et al. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 308, 537–541 (2005).
10. Cramer, C., Fischer, P. & Windhab, E. J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 59, 3045–3058 (2004).
11. Gañán-Calvo, A. M. & Gordillo, J. M. Perfectly Monodisperse Microbubbling by Capillary Flow Focusing. Phys. Rev. Lett. 87, 274501 (2001).
12. Anna, S. L., Bontoux, N. & Stone, H. A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 82, 364–366 (2003).
13. Li, Z., Leshansky, A. M., Pismen, L. M. & Tabeling, P. Step-emulsification in a microfluidic device. Lab. Chip 15, 1023–1031 (2015).
14. Elvira, K. S., Gielen, F., Tsai, S. S. H. & Nightingale, A. M. Materials and methods for droplet microfluidic device fabrication. Lab. Chip 22, 859–875 (2022).
15. Xia, Y. & Whitesides, G. M. Soft Lithography. Angew. Chem. Int. Ed. 37, 550–575 (1998).
16. Aghvami, S. A. et al. Rapid prototyping of cyclic olefin copolymer (COC) microfluidic devices. Sens. Actuators B Chem. 247, 940–949 (2017).
17. Wegrzyn, J. et al. Microfluidic architectures for efficient generation of chemistry gradations in droplets. Microfluid. Nanofluidics 14, 235–245 (2013).
18. Ren, K., Dai, W., Zhou, J., Su, J. & Wu, H. Whole-Teflon microfluidic chips. Proc. Natl. Acad. Sci. 108, 8162–8166 (2011).
19. Durán, I. R. & Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 99, 106–186 (2019).
20. Arunachalam, S. & Mishra, H. Collective wetting transitions of submerged gas-entrapping microtextured surfaces. Droplet n/a, e135 (2024).
21. Salami, T. O. et al. Toward a better understanding of synthesis and processing of ceramic/self-assembled monolayer bilayer coatings. J. Electron. Mater. 34, 534–540 (2005).
22. Owen, M. J. & Smith, P. J. Plasma treatment of polydimethylsiloxane. J. Adhes. Sci. Technol. 8, 1063–1075 (1994).
23. Makamba, H., Kim, J. H., Lim, K., Park, N. & Hahn, J. H. Surface modification of poly(dimethylsiloxane) microchannels. ELECTROPHORESIS 24, 3607–3619 (2003).
24. Xu, J. H., Li, S. W., Tan, J., Wang, Y. J. & Luo, G. S. Controllable Preparation of Monodisperse O/W and W/O Emulsions in the Same Microfluidic Device. Langmuir 22, 7943–7946 (2006).
25. Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 12, 422–433 (2012).
26. Dinh, H.-H.-Q., Santanach-Carreras, E., Schmitt, V. & Lequeux, F. Coalescence in concentrated emulsions: theoretical predictions and comparison with experimental bottle test behaviour. Soft Matter 16, 10301–10309 (2020).
27. Bezerra, M. D. A., Arruda, M. A. Z. & Ferreira, S. L. C. Cloud Point Extraction as a Procedure of Separation and Pre‐Concentration for Metal Determination Using Spectroanalytical Techniques: A Review. Appl. Spectrosc. Rev. 40, 269–299 (2005).
28. Perelomov, L. et al. Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants. Sustainability 16, 4804 (2024).
29. Duran, M. et al. Microcapsule production by droplet microfluidics: A review from the material science approach. Mater. Des. 223, 111230 (2022).
30. Chachanidze, R., Xie, K., Lyu, J., Jaeger, M. & Leonetti, M. Breakups of Chitosan microcapsules in extensional flow. J. Colloid Interface Sci. 629, 445–454 (2023).
31. Pereiro, I., Fomitcheva Khartchenko, A., Petrini, L. & Kaigala, G. V. Nip the bubble in the bud: a guide to avoid gas nucleation in microfluidics. Lab. Chip 19, 2296–2314 (2019).
32. Ghasemzaie, N., Jeyhani, M., Joshi, K., Lee, W. L. & Tsai, S. S. H. ATPSpin: A Single Microfluidic Platform that Produces Diversified ATPS-Alginate Microfibers. ACS Biomater. Sci. Eng. (2024) doi:10.1021/acsbiomaterials.4c00110.
33. Hakim Khalili, M. et al. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 15, 2341 (2023).
34. Sun, X. et al. Facile fabrication of drug-loaded PEGDA microcapsules for drug evaluation using droplet-based microchip. Chin. Chem. Lett. 33, 2697–2700 (2022).
35. Polymers | Free Full-Text | Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. https://www.mdpi.com/2073-4360/15/10/2341 (2024).