Pressure-Controlled Microfluidics in Organ-On-A-Chip Research
Organ-on-Chip (OOC) technology is transforming biomedical research and pharmaceutical development. A key feature of OOC models is controlled fluid perfusion or recirculation. This provides dynamic and physiologically relevant models of organ microenvironments. Among the various flow control methods, pressure-controlled microfluidics stands out for OOC applications due to flow-rate stability and the ability to control shear stress. In this review, we’ll discuss various microfluidic flow control systems as they are applied in OOC models using Fluigent’s solutions.
Microfluidic flow-controllers for organ-on-a-chip applications
Organ-on-chip (OOC) can be used to mimic the structure and function of human organs in a microscale device [1]. OOC devices typically consist of cells or organoids contained in microfluidic channels designed to closely replicate particular organ responses. By recreating the microenvironment and cellular interactions of an organ, OOC technology provides a more physiologically relevant model for studying organ function, disease mechanisms, and drug responses compared to traditional two-dimensional cell cultures or animal models.
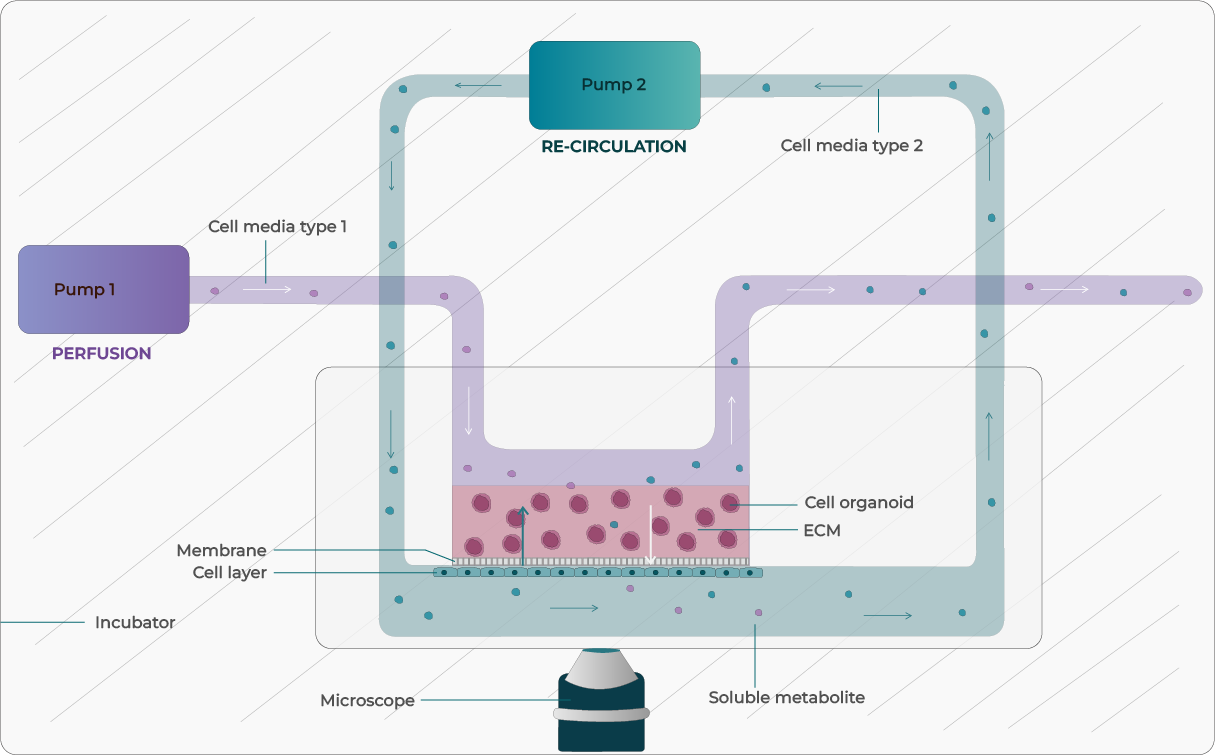
Figure 1: Illustration of OOC with media perfusion and recirculation integration.
One of the main factors involved in replicating the physiological microenvironment in OOC devices is controlled liquid perfusion systems to deliver nutrients and transport drugs, metabolites, and transcriptomic factors. Researchers have access to a variety of flow-control technologies, each having their advantages and their disadvantages (Table 1).
1. Peristaltic Pumps
Peristaltic pumps use positive displacement to squeeze a flexible tube to create a pulsatile flow of liquid through the tube. They are accessible and easy-to-set-up systems used in biological fields, as are is compatible with various types of fluids. They present a low risk of contamination as the liquid only contacts the tubing. Flow rate control and pressure capabilities are low, and the pulsatile aspect may not be suitable for certain applications, such as vascularized models.
2. Syringe Pumps
A syringe pump is a mechanical motor-driven pump that uses one or more syringes for fluids infusion. They are user-friendly, with intuitive control interfaces for programming and adjusting parameters such as flow rate, volume, and infusion rates.
They can provide both continuous and intermittent flow, depending on the programming settings. This feature is useful for experiments or procedures that require specific flow patterns.
Despite their advantages, syringe pumps have a few limitations such as:
- Low flow rate control and responsiveness
- Limited volume of the syringe which will require periodic refilling steps
- An increased risk of contamination as the fluid is in direct contact with the syringe
It is difficult to recycle perfusion media continuously
3. Pressure flow controller
Pressure flow controllers use pneumatic systems to generate pressure and drive the liquid flow through the microfluidic device. Their advantages include: high flow rate stability and fast responsiveness. They can provide continuous or pulsatile flow options and can be integrated into multi-organ systems. Choosing the right perfusion technology depends on your OOC’s specific demands for flow rate, pressure & flow profile, pulsatility, and cell-compatibility. For detailed specifications, see this review.
TABLE 1 : Comparison of different microfluidic technologies used for OOC
| Peristaltic Pump | Syringe Pump | Pressure driven Pump | |
|---|---|---|---|
| Flow stability and precision | Low | Medium | High |
| Time responsiveness | High | Low | High |
| Fluid recirculation | ☑️ | ✖️ | ☑️ L-switch |
| Ability to inject small volumes | Low | High | Medium |
| Sample agitation | ☑️ | ✖️ | ☑️ |
| Possibility to create (program) complex flow profile | ✖️ | ✖️ | ☑️ (LineUp Series) |
Why use pressure control for Organ-on-Chip applications?
Microfluidic pressure control enables researchers to precisely control and recreate the flow rates, shear stresses, and mechanical forces experienced by cells and tissues within the microfluidic device. By closely replicating in vivo physiological conditions, it enhances the reliability and reproducibility of experimental outcomes.
Since pressure is an intrinsic driver of the flow, modulating it allows fine-tuned control over fluid dynamics to establish physiological gradients and deliver targeted mechanical cues that influence cell behavior, differentiation, and function. This capability underpins the creation of complex microenvironments — from precise vascular shear profiles to defined interstitial pressure landscapes.
Precise pressure control is essential for controlled drug delivery and perfusion within the organ-on-a-chip device. It allows for controlled exposure of cells and tissues to drugs, toxins, or other substances, enabling the assessment of their effects on organ function and response.
Considerations for Organ-on-Chip Applications
Media perfusion of OOC devices serves as a circulatory system that maintains a concentration gradient for nutrient and waste convective transport [2]. The choice of the microfluidic liquid perfusion system to connect to the organ-on-a-chip needs to be considered based on several factors:
- Flow rate, pressure control and pulsatility
Reproducing a physiological microenvironment requires mimicking the same conditions in-vivo, depending on the studied organ system. Lung-on-chip models, that aim to mimic the breathing motion and airflow in the lungs, may require low-to-moderate flow rates (a few microliters per minute) and gentle pressure conditions to simulate breathing dynamics and the alveolar microenvironment accurately [3]. Other applications such as heart-on-chip models often require pulsatile flow to mimic the rhythmic contraction of the heart. They may need higher flow rates (tens to hundreds of microliters per minute) and moderate-to-higher pressure capabilities to generate pulsatile flow patterns that replicate the physiological pumping action of the heart.
- Cell viability and shear stress
OOC systems culture living cells and tissues that are sensitive to mechanical forces and fluidic conditions. Excessive shear stress can impact cell viability, proliferation and functionality. It is important to consider the shear stress levels generated by the pump type and select one that minimizes potential damage to the cells while maintaining adequate fluid flow. When working with neuronal cells in brain models that are sensitive to shear stress, it is crucial to carefully control flow rates and avoid high shear stress conditions that could disrupt cell networks or induce cell damage [4].
- Integration and compatibility
OOC models are aiming to replicate interactions between different organs within the human body. For example, liver-on-chip and lung-on-chip may be connected in series to study drug metabolism and toxicity effects. The systems used for each organ model need to be optimized to enable fluid flow and communication between the different chips. Some OOC models may also include pH measurement levels, oxygen control, and electrical activity sensors, hence the importance of considering compatibility with the microfluidic pressure controller. Long-term cultures and imaging/analysis techniques are crucial for any OOC experiment, therefore it is important to consider the compatibility between the microfluidic platform and the Bio-cabinet/imaging/analysis equipment, including positioning, sample accessibility, and optical clarity.
- Contamination risk
Maintaining a sterile environment is essential in in-vitro model development to reduce variability and ensure reproducible results. Some pump designs allow fluid to contact internal components, increasing the risk of contamination. For organ-on-chip (OOC) applications, pumps that minimize or eliminate fluid contact with internal components are preferred.
Understanding microfluidic pressure control
Pressure controller for OOC
In microfluidics systems,, there are these components that work together to enable researchers to simulate dynamic microenvironments, maintain stable conditions for long-term experiments, and precisely control flow direction.
| Component | Function | Role in OOC System |
|---|---|---|
| Pressure sources | Create and regulate the driving force behind fluid movement | Fluid perfusion across tissue/organ models |
| Pressure and Flow Sensors | Monitor internal pressure and flow rate within microchannels | Ensure that physiological pressure/flow conditions are maintained without damaging sensitive cells |
| Pressure controllers | Adjust flow based on sensor feedback to maintain set conditions | Stable, long-term culture and dynamic response to changes in the chip environment (e.g. clogging by overgrowth of cells) |
| Valves | Direct, open, or restrict fluid flow within the microchannels | Enable multiplexing, media recirculation, time-controlled stimulation, and multi-organ interaction studies |
| Feedback Control System (e.g. Software control) | Use real-time data to dynamically adjust pressure or flow | Support pulsatile flow, automated workflows, and consistent simulation of physiological dynamics |
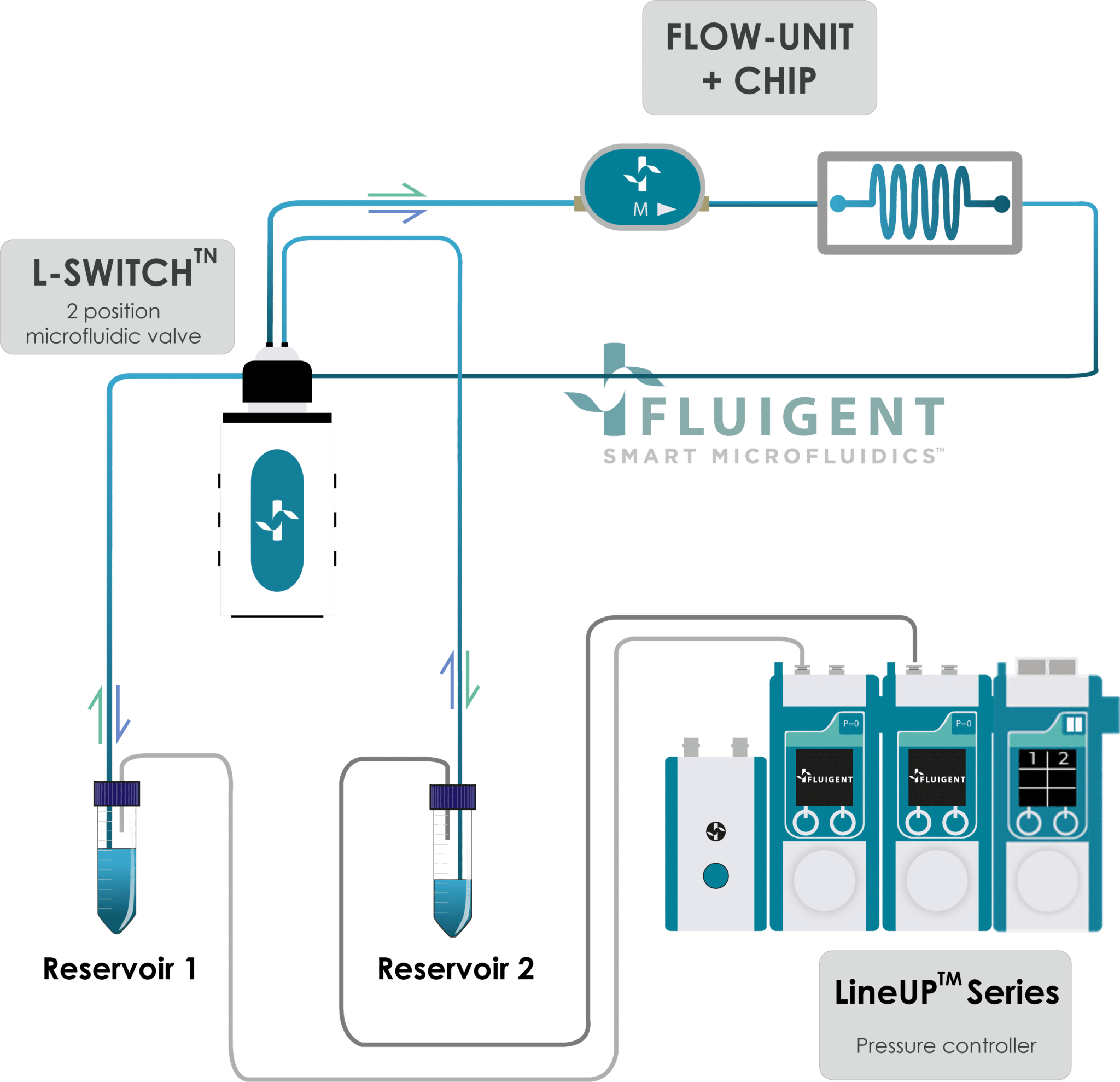
By integrating these components, researchers can effectively use Fluigent’s microfluidic pressure control (Figure 2) to create accurate fluidic environment by recirculating the media in the organ-on-chip.
This setup was tested in collaboration with Beonchip and evaluated against conventional peristaltic pumps to assess the impact of flow stability on cell behavior. Given that endothelial cells are highly sensitive to even minor variations in flow rate, maintaining consistent shear stress is critical for physiological relevance in Organ-on-Chip studies.
Omi, integrated Organ-on-a-chip Fluidic Platform
Building upon Fluigent’s established pressure-based microfluidic systems, the Omi platform represents a modular and integrated solution (with pressure source, sensors and valves) for managing complex fluid handling protocols in Organ-on-a-Chip (OOC) applications.
Designed to extend the functionality of classical Fluigent setups, Omi provides a unified interface for precise pressure regulation, flow control, and automated fluid switching, enabling reproducible execution of protocols such as perfusion, recirculation, injection, and sampling.
Key technical features include:
- Chip compatibility through universal adapters, allowing integration with a broad range of OOC devices
- Remote operation via Wi-Fi and tablet application (Android)
- Cloud-based data storage for streamlined monitoring and experiment tracking
- Programmable fluidic routines suitable for compound testing, including drugs, toxins, and metabolites

Organ-on-chip Applications using Fluigent’s systems
In recent years, there have been remarkable advances in organ-on-a-chip (OOC) models—most notably kidney-on-a-chip [2][8], lung-on-a-chip [3], heart-on-a-chip[5], skin-on-a-chip[6], pancreas-on-a-chip[7], brain-on-a-chip [8], etc. Pressure controlled microfluidics have been instrumental in addressing key challenges in these systems by:
- Providing controlled, directional fluid exposure to better mimic in vivo perfusion
- Establishing gradient flows for precise dosing studies
- Enabling co-culture and spatial layering of multiple cell types to reproduce complex organ architecture
- Incorporating human-derived cells to create more physiologically relevant models than traditional animal systems
Microfluidic OOC platforms are transforming the research in personalized medicine and high-throughput drug screening, single-cell analysis, cell–cell interaction studies, and disease modeling, that in return provides deeper insights into human physiology and the development of more effective therapeutics.
Researchers worldwide have widely adopted Fluigent’s microfluidic pressure control systems to develop and investigate organ-on-chip models.
Blood vessel-on-a-chip
To overcome challenges in reproducing stable flow conditions across multiple vessel-on-a-chip (VoC) models, Valeria Orlova’s team [10] developed a fluidic circuit board (FCB) enabling simultaneous perfusion of up to twelve 3D-VoCs using a unified set of control parameters.
By incorporating Fluigent’s FlowEZ pressure controllers, Link-Up module, and flow sensors, the system ensures consistent wall shear stress and haemodynamic forces—that are critical for maintaining endothelial cell function and vascular integrity.
This multiplexed approach marks a significant step forward in scaling and standardizing 3D vascular models. For a detailed overview of the system and experimental outcomes, read the case study here.
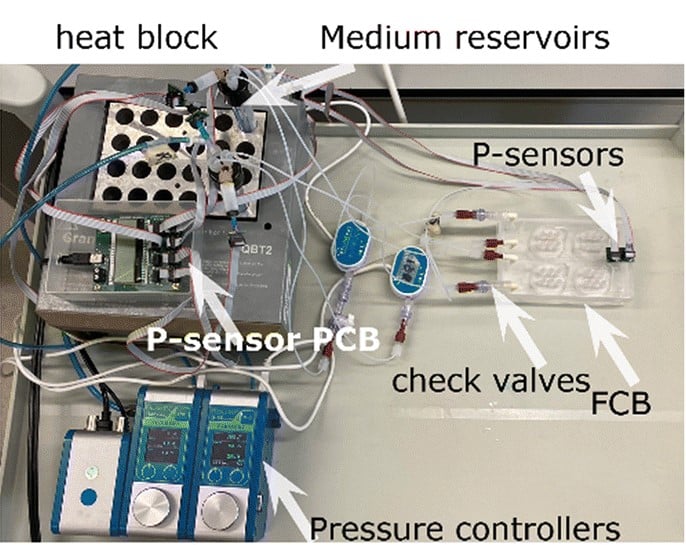
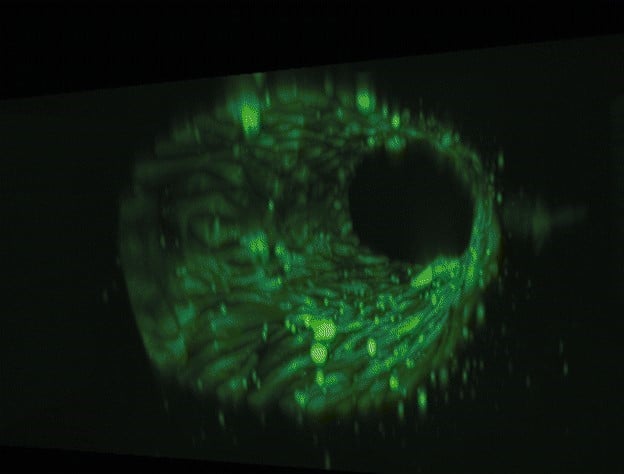
Figure 4: Photograph of the tested fluidic circuit board connected to the external medium reservoirs in a heat block & 3D reconstruction of a blood vessel on a chip.
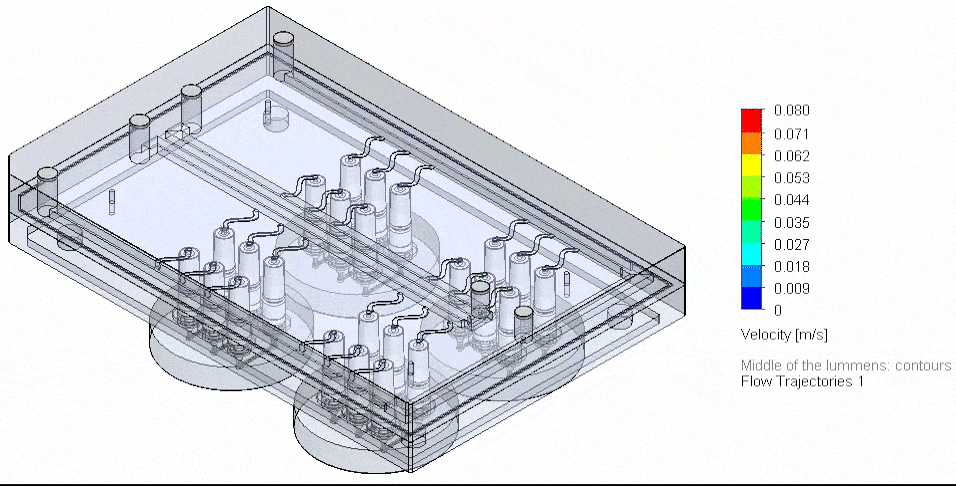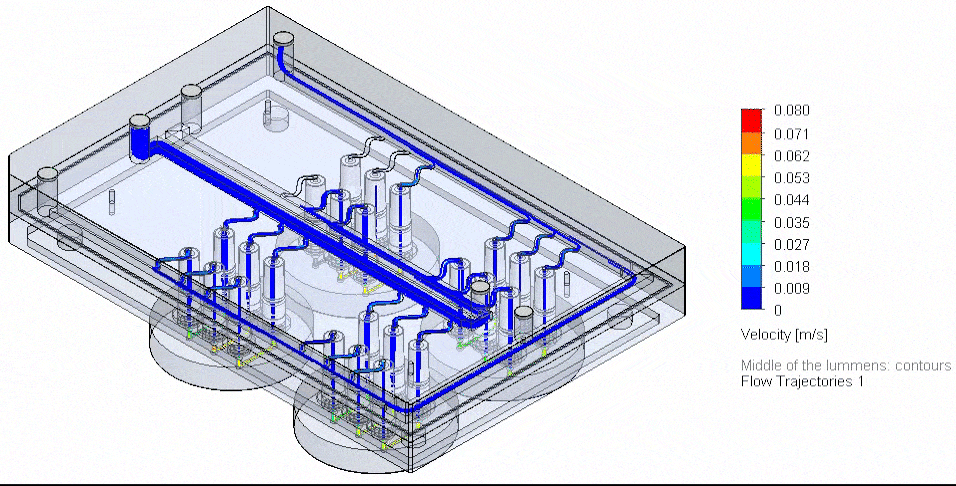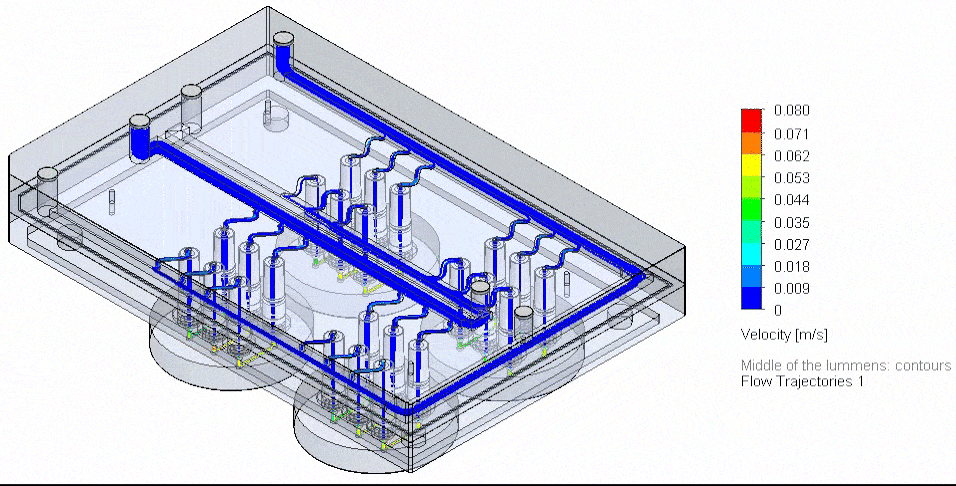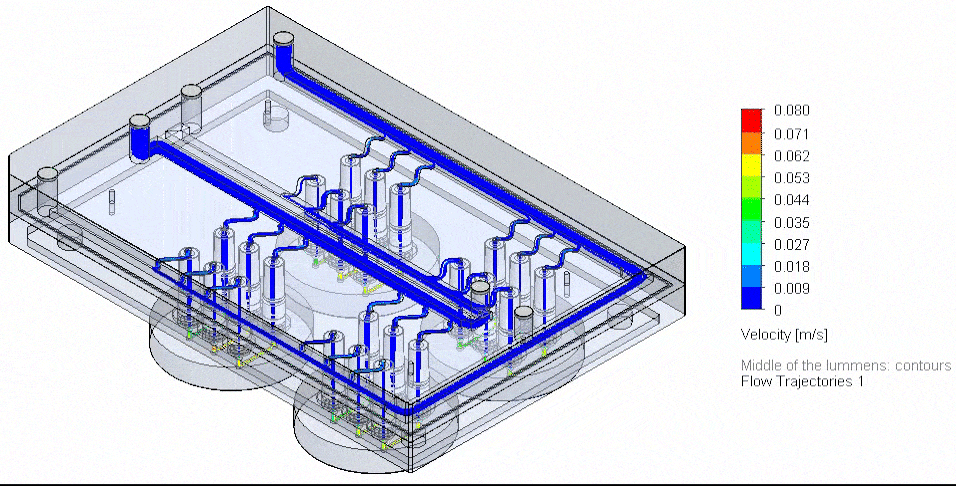
Cancer-on-chip
Van Gent’s team developed a Cancer-on-a-Chip microfluidic platform (Figure 6) for the assessment of patient treatment responses using tumor tissue slices under controlled growth conditions [11]. Their microfluidic pressure control system consists of Fluigent’s MFCS-EZ high-throughput platform. They were able to maintain cell viability and sustain proliferation of breast and prostate cancer PDX tumor slices for up to 14 days. They also studied the tumor slices response to cisplatin chemotherapeutic treatment. Their platform could be used for future studies including biopsies of patient tumors that will be treated with the same chemotherapy. Explore the results and discussion further here. (Microfluidic Cancer-on-Chip Platform by Erasmus MC)
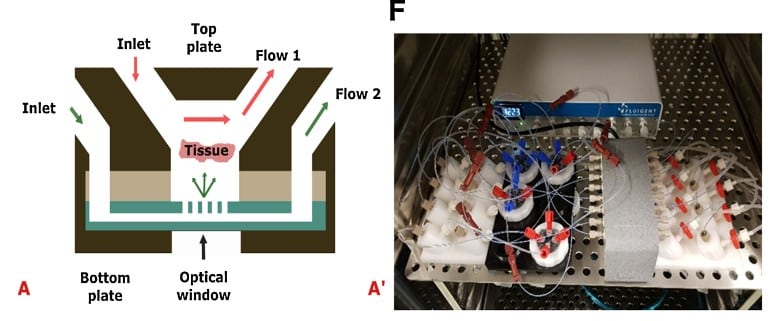
Figure 6: (A) Cross-section of the Cancer-on-a-Chip system illustrating the diffusion and perfusion toward the tissue slice. (A’) The Cancer-on-a-Chip platform connected to a Fluigent Microfluidic flow control system further connected to flowrate sensors (Fluigent FLOW UNIT-S) using Fluigent’s software throughout the culture period.
Cartilage-on-a-chip
Séverine le Gac’s team developed a cartilage on chip model using microfluidic control system (MFCS-EZ, 2 switches and a switchboard) to simulate how chondrocytes react to external stimuli (mechanical or chemical) and to understand processes triggering cartilage diseases like osteoarthritis [12] (Figure 7). This setup notably implements mechanical stimulation on 3D cell cultures and studies the cell response to these stimulations in situ, while also creating dynamic culture conditions. Read the complete application note with details of the model (Complex mechanical stimulation on cartilage-on-a-chip).
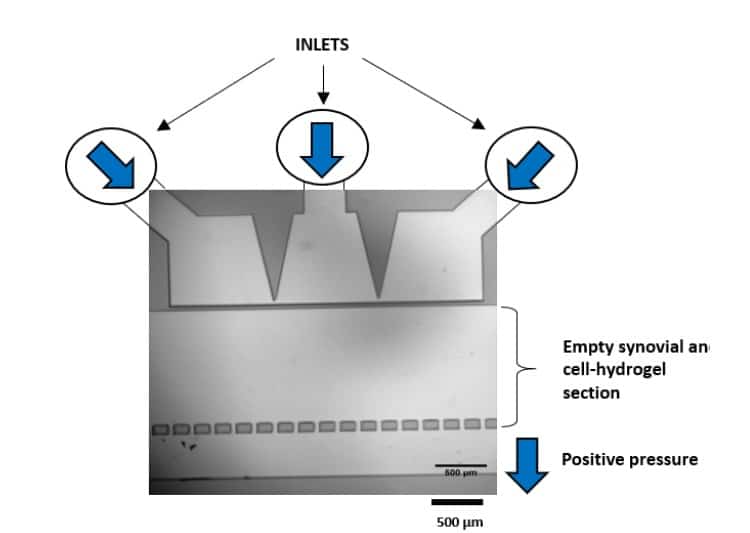
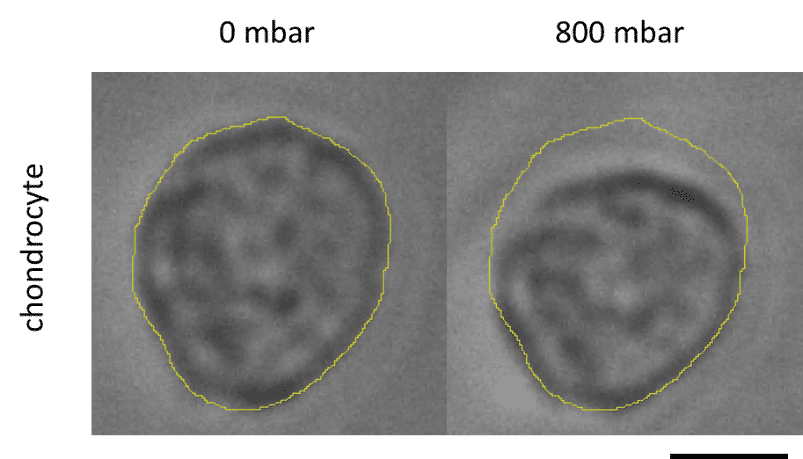
Figure 7: Chondrocyte deformation after mechanical stimulation.
Gut-on-a-chip model
Researchers at the Institut Pasteur de Lille have developed a cost-effective and user-friendly Gut-on-Chip (GoC) platform—the 3DP-µGut—to broaden access to organ-on-chip technologies for gut health and host–pathogen interaction studies.
Unlike conventional GoC systems that rely on costly commercial chips, advanced CAD skills, and cleanroom facilities, the 3DP-µGut is fabricated using a standard SLA 3D printer and freely available design files. This enables medium-throughput production of reproducible, imaging-compatible chips at low cost.
The model was validated using Caco-2 cells, which differentiated into a 3D epithelium after seven days, closely mimicking the native intestinal structure. Its open design allows compatibility with various microfluidic systems, including Fluigent’s Omi integrated platform and FlowEZ pressure controllers, used in the validation process.
To learn more about GoC implementation, flow control strategies, and approaches to modeling host–microbiome interactions:
Webinar: Importance of Flow in Organ-on-a-Chip, featuring the Gut-on-a-Chip Model
Our expert from Institute Pasteur Lille showcase the Gut-on-Chip (GoC) model, supported by the Omi automated platform, highlighting its applications and benefits.
Conclusion
Pressure-controlled microfluidics play a pivotal role in advancing organ-on-a-chip (OOC) research by offering precise, stable, and responsive fluid handling. Compared to other technologies, pressure-based systems better replicate physiological conditions, supporting long-term cell viability, reproducible experiments, and complex flow profiles. Whether simulating vascular shear stress or enabling multi-organ interactions, pressure control ensures optimal performance and reliability in OOC platforms. With solutions like Fluigent’s integrated systems, researchers can now implement customizable setups for their OOC experiments.
Related Products
Expertises & Resources
-
Microfluidics Case Studies A microfluidic Artery-on-a-Chip using Fluigent’s Microfluidic Flow Control System, the MFCS Read more
-
Microfluidics Case Studies Creating a Microfluidic Cancer-on-Chip Platform using Fluigent’s High Throughput Cell Perfusion Pack Read more
-
Microfluidic Application Notes Long-term fluid recirculation system for Organ-on-a-Chip applications Read more
-
Microfluidics Case Studies CNRS/UTC: study of a liver-on-a-chip model Read more
-
Microfluidics Article Reviews Human Blood Brain Barrier (BBB) permeability -on-chip assessment Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Microfluidic Application Notes Development of a human gut-on-chip to assess the effect of shear stress on intestinal functions Read more
-
Expert Reviews: Basics of Microfluidics How to choose a microfluidic chip Read more
-
Microfluidics White Papers An exploration of Microfluidic technology and fluid handling Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
-
Microfluidic Application Notes Cartilage-on-a-chip, an example of complex mechanical stimulation using Fluigent’s technology Read more
-
Expert Reviews: Basics of Microfluidics Mimicking in-vivo environments: biochemical and biomechanical stimulation Read more
-
Expert Reviews: Basics of Microfluidics Flow Control Technologies: Comparison between peristaltic, syringe and pressure pumps for microfluidic applications Read more
-
Expert Reviews: Basics of Microfluidics Microfluidics overview: History and Definition Read more
References
- Leung CM, de Haan P, Ronaldson-Bouchard K, et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers. 2022;2:33. doi:10.1038/s43586-022-00118-6
- Lee SKJ. Kidney-on-a-Chip: a new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr Drug Metab. 2018;19(7):577–583.
- Zamprogno P, Wüthrich S, Achenbach S. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol. 2021;4:168.
- Regmi S, Fu A, Luo K. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep. 2017;7:39975. doi:10.1038/srep39975.
- Liu H, Bolonduro OA, Ning Hu JJ, Rao AA, Duffy BM, Huang Z, et al. Heart-on-a-Chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett. 2020;20(6):2585–2593.
- Lukács B, Bajza Á, Kocsis D, Csorba A, Antal I, Ivan K, et al. Skin-on-a-Chip device for ex vivo monitoring of transdermal delivery of drugs—design, fabrication, and testing. Pharmaceutics. 2019;11(9):445.
- Mun KS, Arora K, Huang Y. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun. 2019;10:3124.
- Raimondi LI, Tunesi M, Comar M, Albani D, Giordano C, et al. Organ-On-A-Chip in vitro models of the brain and the blood–brain barrier and their value to study the microbiota–gut–brain axis in neurodegeneration. Front Bioeng Biotechnol. 2020;8:435.
- Menéndez AC, Du Z, van den Bosch TPP, Othman A, Gaio N, Silvestri C, et al. Creating a kidney organoid vasculature interaction model using a novel organ-on-chip system. Sci Rep. 2022;12(1):20699
- de Graaf MNS, Vivas A, Kasi DG, van den Hil FE, van den Berg A, van der Meer AD, Mummery CL, Orlova VV. Multiplexed fluidic circuit board for controlled perfusion of 3D blood vessels-on-a-chip. Lab Chip. 2023; 23:68-181.
- Chakrabarty S, Quiros-Solano WF, Kuijten MMP, Haspels B, Mallya S, Lo CSY, et al. A microfluidic cancer-on-chip platform predicts drug response using organotypic tumor slice culture. Cancer Res. 2022;82(3):510-520
- Paggi CA, Hendriks J, Karperien M, Le Gac S. Emulating the chondrocyte microenvironment using multi-directional mechanical stimulation in a cartilage-on-chip. Lap Chip. 2022;22(9):1815-1828