Flow-EZ and SliceChip for Pancreas on a Chip Study
SliceChip is an innovative microfluidic platform designed to culture and analyze pancreatic tissue slices. Traditional static ex-vivo culture methods have limiting viability and functionality of the tissue over extended periods due to:
- inadequate oxygenation
- accumulation of waste products
- degradation due to digestive enzymes
To address these challenges, Dr. Agarwal’s Physiomimetic Microsystems Laboratory (PML) developed the microfluidic pancreas-on-chip platform that can provide controlled oxygen and perfusion environments for viability and real-time imaging.
Addressing the Ex-vivo Culturing with Microfluidic Platform
Utilizing enzymatically isolated human pancreatic islets has been an established standard to investigate diabetes pathogenesis and develop new treatments. However, these models do not fully reflect the complex structure of the pancreas and fail to mimic the full exocrine and endocrine changes associated with diabetes. Living pancreatic tissue slices have provided a more comprehensive model, preserving the natural exocrine and endocrine interactions.
The main challenge lays in maintaining the cultures in long-term. The slices have a high oxygen demand as metabolic byproducts and enzymes accumulate, necessitating frequent media changes. Functional studies, such as glucose-stimulated insulin secretion (GSIS) assays, require multiple slices in open wells which increases the risk of contamination and complicates the tracking of individual slices over time. As a solution, microfluidic organ-on-chip platforms replicate physiological oxygenation and perfusion conditions, supporting slice viability and enabling functional and immunolabelling assays analysis.
Design Approach for Pancreas Slices on A Chip
Traditional PDMS-based organ-on-a-chip (OoC) devices have limitations such as gas permeability, drug absorption, and difficulty in handling large tissue samples. SliceChip is designed with Bio-inert, non-absorbent PMMA material to prevent drug interactions. It also features:
- Optimized convective fluid flow for better nutrient delivery.
- Reversible assembly for easy loading and retrieval of slices by Micronut clamp
- Integrated fluidic control for precise oxygen regulation and bubble-free operation.
These characteristics allow long-term culture, GSIS functional assessments, and real-time imaging of primary human and murine pancreatic slices.
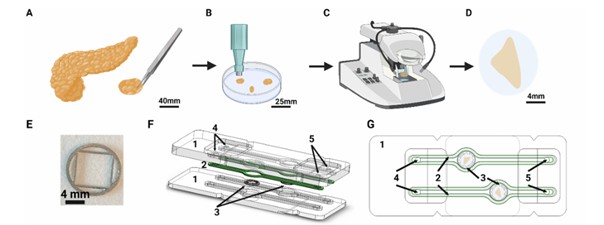
Figure 1: SliceChip of ex-vivo pancreas assembly
The Figure 1 captures the process of slicing the pancreas sample and assembling it into the microfluidic chip:
(A) A section of tissue is removed is taken from the donor pancreas.
(B) The tissue is divided into smaller pieces, cleaned, and suspended in an agarose solution. Once solidified, a biopsy punch extracts a 7 mm diameter cylinder.
(C) A vibratome sectioned the tissue cylinder into 120 μm thick, 7 mm diameter slices.
(D) The pancreatic slices are transferred to a culture dish before being placed into the chip.
(E) Biocompatible 3D-printed stainless steel 316L to anchor the tissue slice.
(F) An exploded view and (G) an assembled top of the SliceChip system illustrate its components:
[1] Milled acrylic chip,
[2] Silicone gasket,
[3] Slice anchor within an 8 mm diameter central culture well
[4] Fluidic inlet,
[5] Fluidic outlet.
Optimization of Fluid Dynamics in OoC by Computational Modelling
Computational models were performed using COMSOL software to optimize fluid dynamics within the SliceChip platform. The analysis focused on:
- Determining the optimal flow rate for glucose washout time. This enabled accurate GSIS experiments while maintaining high time resolution for sample collection.
- Modelling fluid velocity, pressure, and shear stress to confirm that the system provides a uniform and physiologically relevant microenvironment for pancreatic slices.
- Preventing excessive shear stress that could damage islets by ensuring flow rates remain within safe biological limits.
Simulations tested various flow rates (20–80 μl/min), selecting 80 μl/min to ensure a total inlet-to-outlet time of 2.95 minutes. This was shown to maintain high time resolution and allow for discrete sample collection.
At 80 μL/min the simulations generated velocity, pressure, and shear stress profiles:
- Velocity profile: Slow fluid flow around the pancreatic slice (<200 μm/s) despite faster flow in inlet/outlet channels.
- Pressure profile: Uniform gradient across the chip.
- Shear stress: Peaks at 1.2 mPa, staying well below damage-inducing levels for islets.
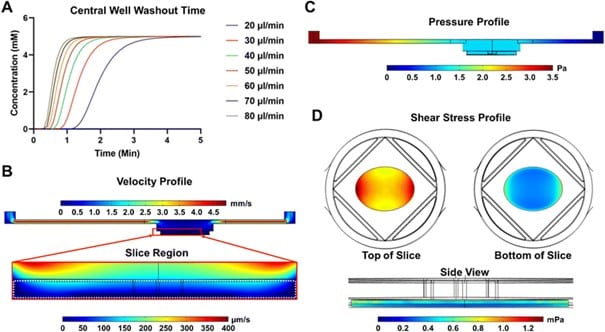
Figure 2: SliceChip Computation Model of Fluidic Parameters Simulated in CAMSOL. (A) Glucose washout time for various flowrates to determine the time necessary for the chamber to match the input stimulant concentration. To ensure that fluid washed through the entire system, including external tubing, in under 3 minutes, a flow rate of 80 μl/min was selected. (B) The fluid velocity profile, at 80 μl/min, is shown with a zoomed in region depicting the area around a modeled pancreatic slice, demarcated by a white dashed line. (C) The pressure profile of the system. (D) The shear stress on the modeled slice: top-down view (pictured left), bottom-up view (pictured right), and side view (pictured bottom).
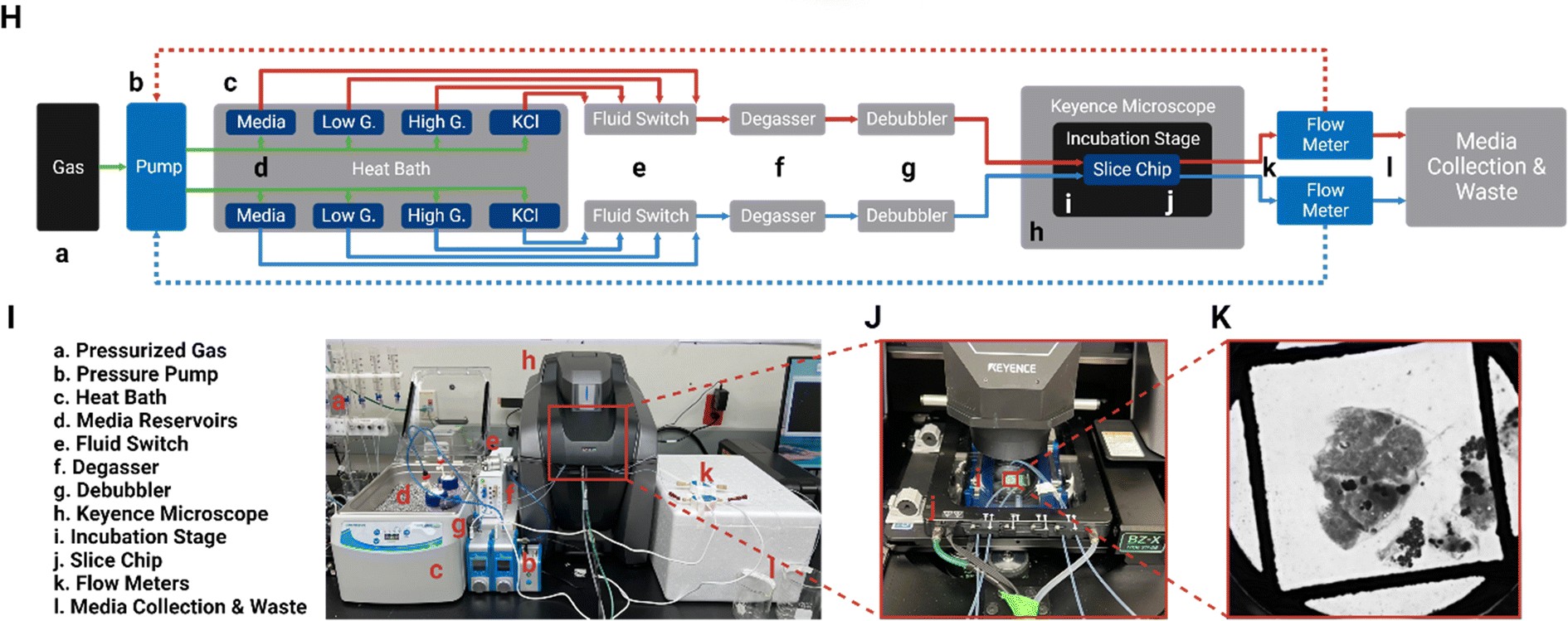
Complete Ex-Vivo Perfusion System Set-Up with Flow EZ
All components, including tubing, bubble traps, and flow units, were sterilized before assembly to maintain a contamination-free environment. Media bottles were connected to an analytical selector valve, allowing controlled perfusion through the system. The microfluidic chip was loaded with pancreatic slices, secured with slice anchors, and placed inside a stage-top incubator on a Keyence BZ-X810 microscope. A Flow EZ pressure controller and Flow Unit ensured the precise media flow.
To prevent gas supersaturation and bubble formation, a degasser was used to remove dissolved gases. This setup generated two levels of residual oxygen in separate flow channels. An optical oxygen sensor inside the chip continuously measured oxygen levels every minute for 16 hours using a multichannel oxygen meter.
The Fluidic System Allows for:
- Automated switching between media reservoirs
- Precise regulation of dissolved oxygen levels
- Bubble-free and leak-free operation for extended slice culture
Figure 3 illustrates the complete set-up:
(H) A block diagram of the fluid pathway within the pancreatic SliceChip system shows the,
[a] pressurized O2 and CO2 gas tanks that provide the pressure source for, [b] the Fluigent pressure pump,
[c] heat bath,
[d] pressurized media reservoirs that contain basal media for long term culture as well as low glucose, high glucose and KCl solutions for GSIS assay,
[e] in-line fluid switches for switching between solutions from
[d] Media exiting the reservoir is passed through,
[f] a degasser,
[g] a de-bubbler to control oxygenation levels and eliminate large bubbles within the fluid lines.
[h] Keyence fluorescent microscope
[i] Modified incubator stage that maintains the temperature of the chip and limits environmental exposure holds,
[j] the SliceChip is clamped using a Micronit clamp for long term culture, imaging, and functional assays.
[k] Flow meters monitor the flow rate and act in a continuous feedback loop with the regulators to modulate pressure and ensure a consistent flow rate before,
[l] effluent media is collected.
(I) A picture of the SliceChip and the fluidic control system with, (J) a closeup view of the incubation stage within the Keyence and (K) a brightfield image of a human slice and anchor within the system as taken from the microscope.
Pancreas Tissue Ex-Vivo Endocrine and Exocrine Functions Assessment
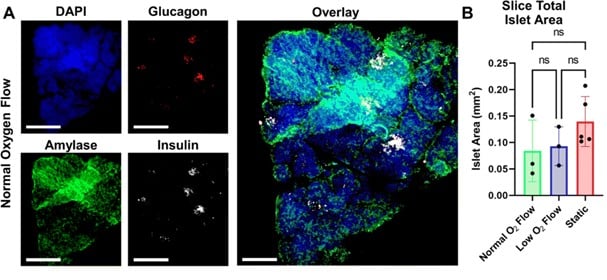
Imaging analysis demonstrated the preservation of both endocrine and exocrine pancreatic tissues in SliceChip-cultured slices.
- Preservation of Pancreatic Tissue Subtypes: Insulin-producing β-cells, glucagon-producing α-cells, and amylase-containing exocrine cells remained present after culture.
- Islet Architecture Maintained: Insulin and glucagon colocalization were observed, consistent with normal islet structure, though enhanced by 3D imaging techniques.
- Islet Viability Confirmed: Continued insulin secretion in response to high glucose suggests that islets remained functional.
- Exocrine Function Assessment: Carbachol stimulation tested exocrine activity, but secreted amylase levels were below detection limits.
- Exocrine Tissue Integrity: Despite low measurable enzymatic activity, immunostaining confirmed amylase presence, indicating exocrine tissue structures were preserved.
Figure 4: (A) Fluorescent Imaging: A pancreatic slice under normal oxygen conditions was immunostained for DAPI (nuclei), glucagon, amylase, and insulin, with images shown as full-focus Z-stack composites. Scale bars: 1 mm for individual stains, 500 μm for the co μm for the composite image. (B) Islet Area Analysis: Total islet area per slice was measured, with static cultures using 2–3 slices per condition, while SliceChip used 1 per run. Statistical analysis showed no significant difference in islet area across conditions, though this does not reflect insulin secretion function or overall slice viability.
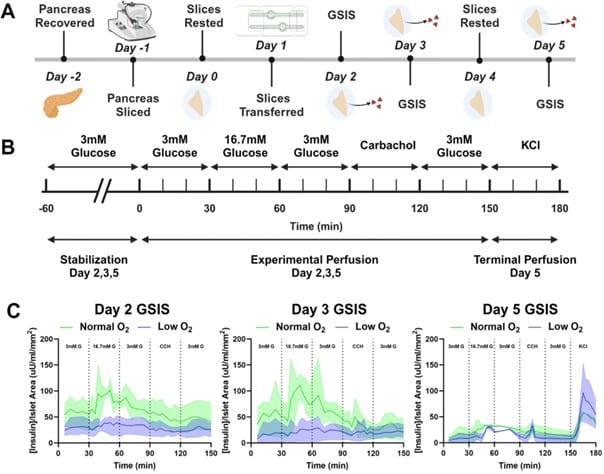
GSIS Ex-vivo On-Chip Evaluation
Traditional GSIS experiments are endpoint-based, requiring separate slices for each test. SliceChip enables repeated GSIS assessments on the same slice over multiple days while maintaining sterility. It also allows simultaneous testing under different oxygen conditions to analyze the impact on insulin secretion. In the Figure 5, insulin secretion is normalized to islet area. Solid lines represent mean responses while shaded regions show variability.
Figure 5: (A) Experimental Timeline: Outlines the full experiment from pancreatic slice procurement to final perfusion on day 5, including GSIS assessments and resting periods. (B) GSIS Testing Protocol: Details the glucose perfusion schedule, including stabilization, sequential glucose and carbachol stimulations, and a terminal KCl perfusion on day (C) Insulin Secretion Response: Shows insulin secretion time curves under normal and low oxygen conditions, with responses normalized to islet area and compared across days 2, 3, and 5.
- Day 2 & 3 Findings: Slices in normal oxygen display a physiological insulin spike in response to glucose, whereas low oxygen slices show a diminished response.
- Day 5 Findings: Both conditions exhibit an insulin spike, though the amplitude is reduced compared to earlier days.
Conclusion
The novel ex-vivo pancreas culturing approach allows for long-term culturing and viability. GSIS assessments, yielded robust insulin secretion studied that may be normalized to islet area and measured over multiple days. Early experiments show that slices under normal oxygen conditions generate a physiological insulin spike in response to glucose, while those in low oxygen exhibit a blunted response. For full results and discussion on serial ex-vivo perfusion and pancreas on chip technology, please see the full paper.
Related Products
Expertises and resources
-
Microfluidic Application Notes Gut-on-Chip Model Development Using OOAC Platform, Omi Read more
-
Microfluidic Application Notes Long-term fluid recirculation system for Organ-on-a-Chip applications Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Microfluidic Application Notes Development of a human gut-on-chip to assess the effect of shear stress on intestinal functions Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
-
Expert Reviews: Basics of Microfluidics Selecting Microfluidic Tubing Read more