Drug-Loaded Liposome Preparation Using Microfluidics
Amiodarone, a drug currently known as an antiarrhythmic agent, shows potential in targeting ovarian cancer by inhibiting CPT1A, a protein involved in fatty acid oxidation. However, toxicity issues have hindered its usage potential. In this study published in Scientific Reports (2024), a microfluidic approach was used to develop drug delivery systems, including liposomes and amiodarone particles, to reduce side effects and toxicity. The optimization of liposome production parameters (flow rate, temperature, concentrations) and stability evaluation were conducted using Fluigent flow controllers and a staggered herringbone mixer. In vitro tests confirm the efficacy of these drug delivery systems.
A Paper From the Ca’Foscari University of Venice
Saorin, A.; Saorin, G.; Duzagac, F.; Parisse, P.; Cao, N.; Corona, G.; Cavarzerani, E.; Rizzolio, F. Microfluidic Production of Amiodarone Loaded Nanoparticles and Application in Drug Repositioning in Ovarian Cancer. Sci Rep 2024, 14 (1), 6280. https://doi.org/10.1038/s41598-024-55801-3
This article results from the work of the Department of Molecular Sciences and Nanosystems (Ca’Foscari University of Venice), which conducts interdisciplinary research across three main areas: nanosystems and nano-biomaterials for various applications (biomedical, sensory, environment, energy), sustainable chemistry for industry and the environment, and new technologies for studying cultural heritage products. With the diverse expertise of its staff, the department aims to address key challenges in these fields while collaborating with local businesses and industries.
Ovarian Cancer and the Involvement of Fatty Acid Oxidation
How Ovarian Cancer Is Defined
Ovarian cancer ranks as the third most prevalent gynecological cancer following cervical and uterine cancers. Despite its relatively lower occurrence, it holds the highest mortality rate among gynecological cancers and ranks fifth in terms of cancer-related deaths in women.
There are more than 30 different ovarian cancer types, with epithelial ovarian cancer comprising the majority (90%) (Figure 1). Among its subtypes, high-grade serous ovarian cancer is the most frequently diagnosed, accounting for 70% of cases. Typically detected at an advanced stage (Figure 1), it exhibits a dismal five-year survival rate of only 30%, caused by tardive diagnosis. 1,2
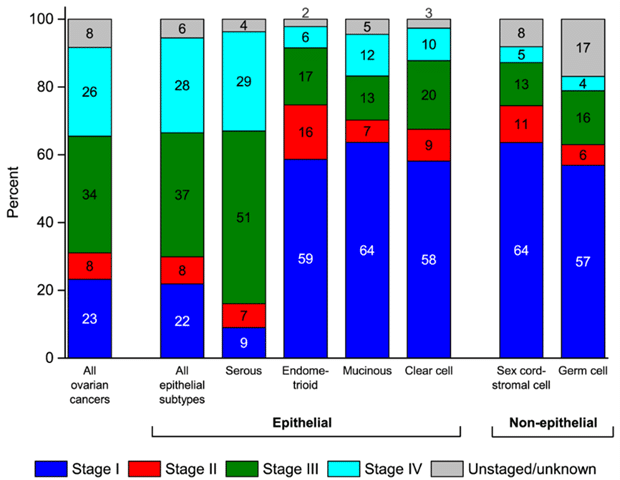
Figure 1: Stage distribution (%) for ovarian cancers.2
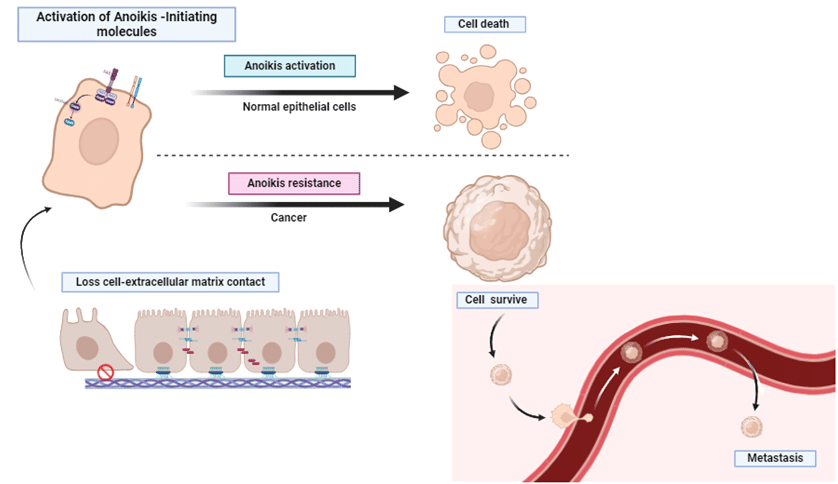
Anoikis Resistance Via Fatty Acid Oxidation
The spread of epithelial ovarian cancer depends on tumor cells developing the capability to withstand anoikis, a process enabling them to survive and attach to metastatic sites in the peritoneal cavity or endure within ascites (Figure 2). Resistance to anoikis triggers a metabolic shift toward a preference for fatty acid oxidation, facilitated by the upregulation of a component of the carnitine palmitoyltransferase system (CPT).
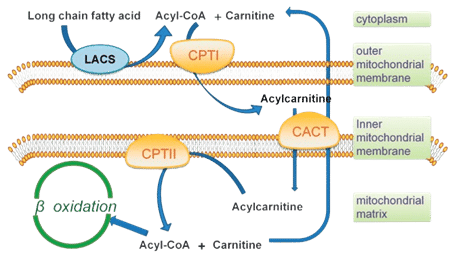
CPT is responsible for transporting long-chain fatty acids across mitochondrial membranes, comprising CPT1, CPT2, and CACT (Carnitine AcylCarnitine Translocase) as presented below (Figure 3). While CPT2 and CACT are in the inner mitochondrial membrane, CPT1 resides in the outer membrane and is crucial for initiating fatty acid oxidation. It exists in three isoforms: CPT1A (liver form), CPT1B (muscle form) and CPT1C (brain form). Ovarian cancer cell lines have demonstrated dependency on CPT1A-mediated fatty acid oxidation for cell cycle progression, as its inactivation decreases ATP levels and induces cell cycle arrest, suppressing growth.
Therefore, targeting the upregulation of fatty acid oxidation, particularly through inhibition of CPT1A, holds promise as a potential therapeutic approach in the treatment of epithelial ovarian cancer.
Amiodarone as a Promising CPT1A Inhibitor
Amiodarone is already used in therapy as an antiarrhythmic agent. It was approved by the FDA (Food and Drug Administration) in 1985 for several diseases (such as atrial fibrillation and arrhythmia). Recently, preclinical studies suggested the potential of amiodarone as an anticancer agent, particularly in epithelial ovarian cancer. However, its repurposing is limited by significant toxicity, primarily due to its lipophilicity, leading to prolonged accumulation in various tissues. Given the importance of drug repurposing and the high toxicity of other proposed CPT1A inhibitors, there’s interest in developing delivery systems to minimize amiodarone’s off-target effects. Among these delivery systems, liposomes have shown efficacy as drug carriers.
Liposomes as Efficient Nanocarriers for Drug Delivery
Liposomes are spherical vesicles composed of phospholipid bilayers. They can encapsulate both hydrophilic and hydrophobic active pharmaceutical ingredients (API), making them highly promising for drug delivery. The organization in vesicles and the encapsulation of molecules is due to lipid properties: hydrophobic tail and hydrophilic head. Hydrophobic tails cannot be in contact with the aqueous phase and consequently shape themselves in a vesicle. 5
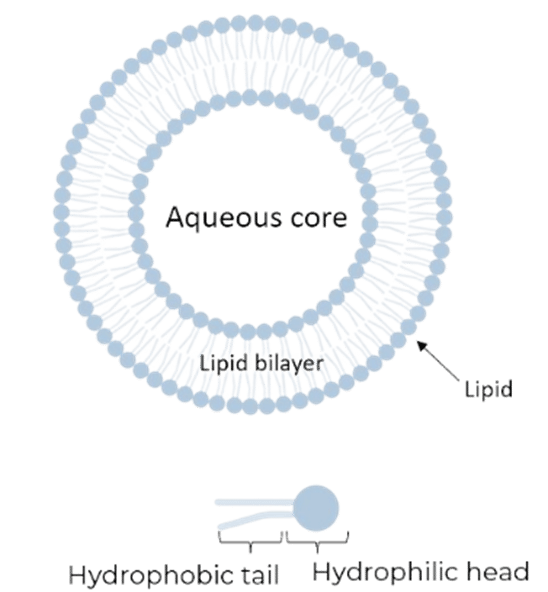
Figure 4: Simple liposome and lipid representations.
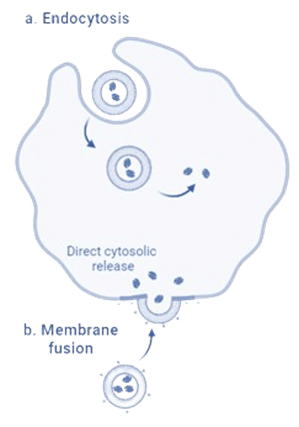
Figure 5: Internalization of liposome.6
In addition, the surface modification of liposomes with moieties, like poly (ethylene glycol) (PEG), antibodies, or protein conjugates, enable targeted delivery, reducing the dosage needed and limiting delivery to non-essential areas. In the case of drug delivery, liposomes can be internalized in two ways: endocytosis or membrane fusion, as described in Figure 5. This approach facilitates a prolonged release of APIs.
The physical characteristics of liposomes, such as size, polydispersity index (PDI), and lamellarity, are influenced by lipid composition and the preparation method. Traditional methods like thin-film hydration and extrusion have limitations, including being time-consuming, having high batch-to-batch variation, and difficulties in scaling up. Microfluidics emerged as a promising technique for manufacturing liposomes, offering high-quality mixing and improving size control and homogeneity.
Research Goals
In this study, researchers from the Department of Molecular Sciences and Nanosystems (Ca’Foscari University of Venice) presented a detailed protocol for amiodarone-loaded liposome synthesis. They demonstrated the effects of amiodarone encapsulation in ovarian cancer cells and amiodarone’s efficacy as a CPT1A inhibitor.
Different parameters (flow rate ratio, temperature, etc.) were tested to measure the impact on liposome production (liposome size, size distribution, encapsulation efficacy, etc.) using a staggered herringbone mixer and Fluigent’s pressure controller (Flow EZ) to maintain a precise flow rate.
This study is the first to investigate amiodarone’s potential in ovarian cancer inhibition of CPT1A and load it into liposomes.
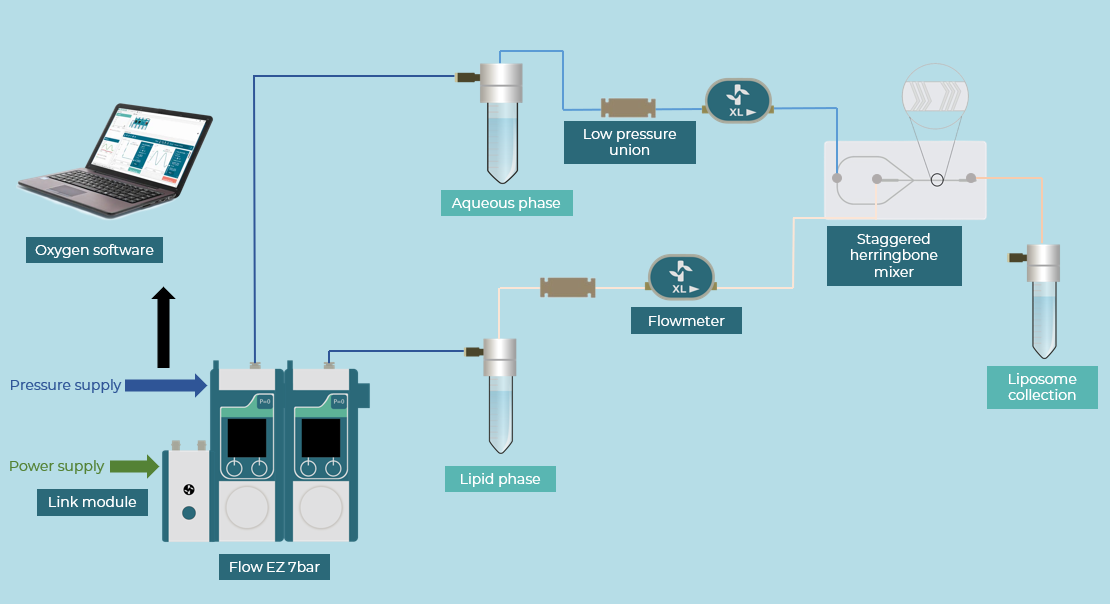
How to Prepare a Microfluidic Setup for Monodisperse Liposome Preparation
A microfluidic setup was used to produce both empty and amiodarone-loaded liposomes, based on Doxil® formulation (HEPC: CHO: DSPE-PEG). The setup included two Fluigent pressure-driven pumps (Flow EZ – 7 bar), two flow controllers (Flow Units, Range XL), and a micromixer glass chip controlled by Oxygen, Fluigent’s software for protocol automation. The micromixer chip combined a co-flow and staggered herringbone structures, which help control the flow inside the chip with high precision.
To produce empty liposomes, the Doxil formulation, approved by the FDA (Food and Drug Administration), was dissolved in a specific molar ratio in ethanol and mixed in the chip with Dulbecco’s Phosphate-Buffered Saline (DPBS). Following the same protocol, amiodarone-loaded liposomes were created by adding amiodarone to the organic phase as it is a hydrophobic molecule.
Various flow rate ratios and temperature conditions were tested, with different amiodarone concentrations for the liposomes. Samples collected were dialyzed overnight in DPBS to remove free molecules and ethanol. Additionally, a separation protocol involving centrifugation was followed to separate the liposomes from non-encapsulated amiodarone particles.
The liposome size was measured with DLS (Dynamic Light Scattering) and TEM (Transmission Electron Microscopy) techniques.
Different human ovarian cancer cells (A2780, Kuramochi, OVCAR-5, and SKOV3) were studied to evaluate the amiodarone and drug delivery systems effects. Cell splitting was performed at 70–80% confluency using trypsin, and cell morphology and growth were monitored daily under a microscope. Suspension cultures, mimicking anoikis-resistant cells, were obtained by coating plates with a Poly-HEMA solution. SKOV3 suspension cultures formed spheroids spontaneously, making them a suitable model for evaluating drug efficacy and penetration in ovarian cancer metastasis.
Testimonial

“One of the research group’s focuses was the study of ovarian cancer. Leveraging Fahriye Duzagac’s (PostDoc) previous experience and Asia Saorin’s (PhD student) freshly written review on ovarian cancer biomarkers, it was a natural progression to develop a project aimed at testing amiodarone as an inhibitor of CPT1A. However, the formulation developed using microfluidics was crucial in unlocking the drug’s full potential. This advancement was thanks to Gloria Saorin (PhD student), who was working with the Fluigent system for the production of liposomes. Thanks to the microfluidic approach it was possible to optimize particles production parameters with better control over them, using smaller amounts of formulants and drug i.e. saving time, money and allowing an improvement of the sustainability of the process.”
Results: Enhanced Efficacy of Amiodarone Against Epithelial Ovarian Cancer Models Through Microfluidic Encapsulation
Before loading amiodarone, the microfluidic production process was optimized by studying the effects of temperature and flow rate ratios (FRR) on liposomes while assessing their long-term stability.
To find the best temperature and its impact on liposome size and lipid composition, different conditions were tested:
- RT_RT: each phase reservoir was at room temperature.
- 63_63: both phase reservoirs were heated at 63°C.
- 63_RT: only the ethanol reservoir was heated at 63°C, the other one remained at room temperature.
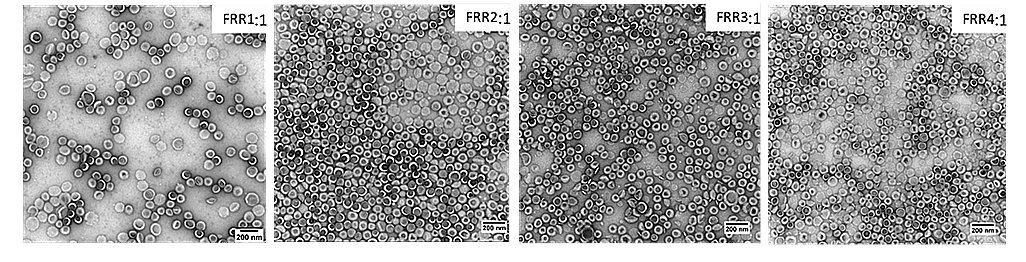
TEM and DLS analysis showed that liposomes were bigger when reservoirs were both heated (figures 7-8). Liposomes were also bigger when both reservoirs were at RT and only the ethanol reservoir was heated. Among the temperature conditions tested, heating only the alcoholic solution reservoir (63_RT) emerged as the most reproducible method based on the lipid yield (determined by H-NMR), avoiding flow instability and solvent evaporation.
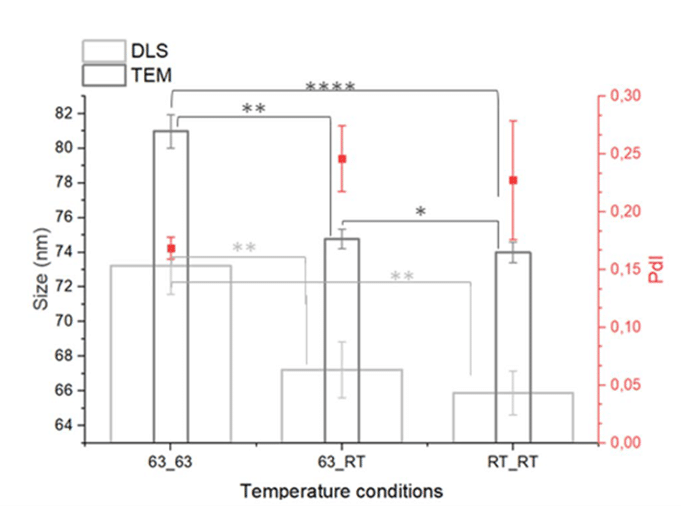
Figure 7: Impact of temperature on liposome size.
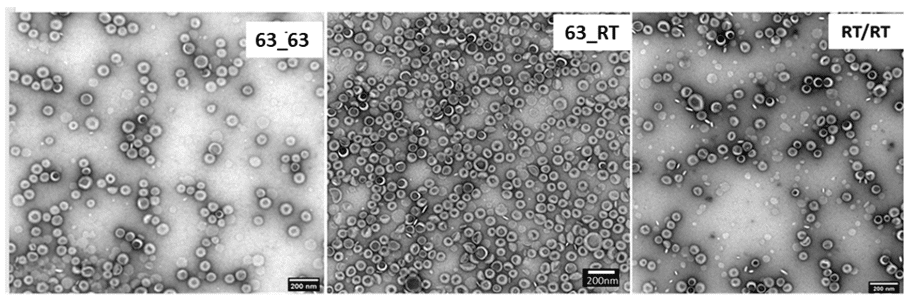
Figure 8: TEM images obtained for each tested temperature.
After, the impact of the flow rate ratios (FRR) on liposome size was evaluated.
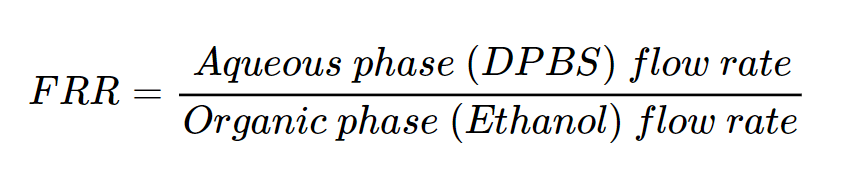
The FRR was varied (4:1, 3:1, 2:1, 1:1) while keeping the other studied parameters constant (total flow rate = 1 mL/min, temperature 63_RT, lipids 10 mM). For instance, at TFR = 1 mL/min and FRR = 3:1, the flow rate was 750 µL/min for the aqueous phase and 250 µL/min for the organic phase. Analysis using DLS and TEM indicated that FRR values of 4:1, 3:1, and 2:1 yielded liposomes with favorable characteristics, with the optimal polydispersity index (PdI) observed at FRR 2:1. A decrease of the liposome size was observed with increasing FRR (Figures 9-10).
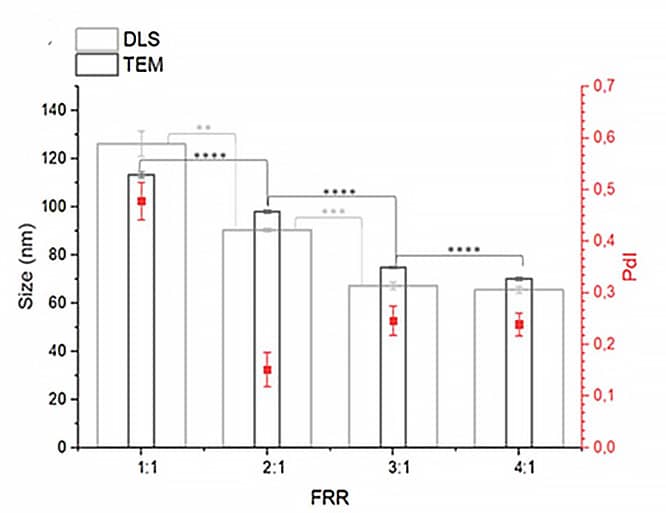
Figure 9: Impact of FRR on liposome size.
Following these initial characteristics, the appropriate microfluidic liposome production parameters were selected before encapsulating the amiodarone (Table 1).
Table 1: Parameters selected for amiodarone encapsulation in liposome
| Temperature | 63_RT |
| TFR (Total Flow Rate) | 1 mL/min |
| FRR (Flow Rate Ratio) | 3:1 |
| Lipid concentration | 10mM |
| Amiodarone concentration | between 5 and 10 mM |
The encapsulation efficacy (EE) of amiodarone was calculated as follow:
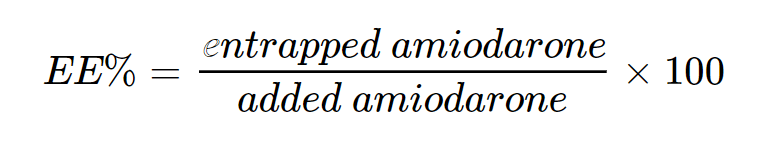
With an amiodarone concentration of 5mM, EE was evaluated at 21 ±4 %, with the hypothesis that amiodarone is in the lipid bilayer of the liposomes as it is known for its insolubility and hydrophobic nature.
Next, the stability of amiodarone-loaded liposomes was measured after one month in storage at 4°C. Results presented a slight increase in the liposome diameter and a stabilization of PdI over time, proving their stability without any aggregations.
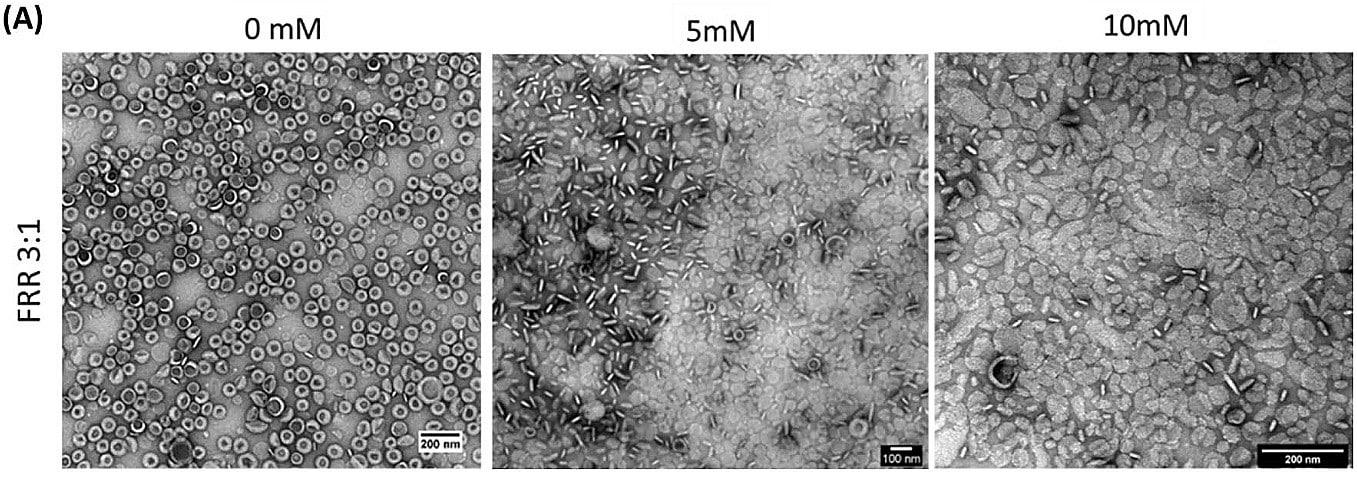
Figure 11: TEM images of the amiodarone-loaded liposomes for different concentrations of amiodarone.
To check their internalization in ovarian cancer cells (A2780), the produced liposomes were stained by rhodamine B, a fluorescence dye. A total internalization of liposomes in the cytosol was observed, as confirmed by the red fluorescence coming from the cytosolic compartment of cells in Figure 12.
Finally, the cell viability rate was measured by the Presto Blue® test for suspension cells and by the CellTiter-Glo® Luminescence assay for adhesion cells. Produced by an FFR3:1, an amiodarone concentration of 5 mM, and a temperature of 63_RT, amiodarone-loaded liposomes were effective in adhesion culture, reducing IC50 values for Kuramochi and OVCAR-5, while A2780 IC50s were like those of the free drug (Figure 13). However, they exhibited lower activity in suspension. Based on these results, liposomes could be therefore optimized for intravenous injections, rather than intraperitoneal injections, followed by an enhanced permeation and a retention effect accumulation in the cancer site.
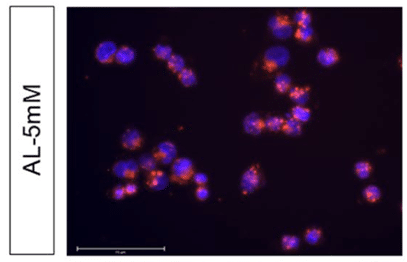
Figure 12: Fluorescence microscopy of A2780 with liposomes labeled with rhodamine B (red).
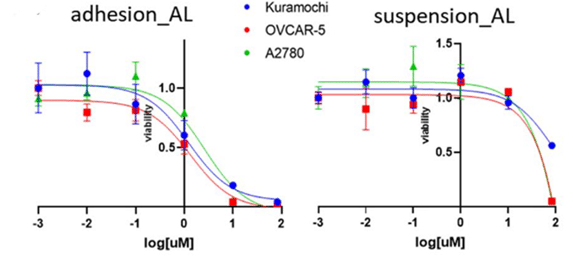
Figure 13: Concentration-viability plot of liposomes with amiodarone in adhesion and suspension culture.
Conclusion
In this paper, researchers from the Department of Molecular Sciences and Nanosystems (Ca’Foscari University of Venice) presented a microfluidic method for drug-loaded liposome production using Fluigent’s liposome production pack, including Flow EZs and Flow Units. It permits obtaining the desired size, an optimal size distribution, and a high drug content. The amiodarone liposomes were effective against epithelial ovarian cancer models, with a possible role in the inhibitory activity of CPT1A. Amiodarone showed promise in reducing cell viability in adhesion culture, although its effectiveness was lower in suspension cultures. Amiodarone particles became stronger with liposomes, and instead proved their capability to inhibit suspension culture growth. Further research, including in vivo studies, will be useful to fully evaluate the clinical potential of these drug delivery systems.
Related Products
Expertises and resources
-
Microfluidics Article Reviews Solid lipid nanoparticles for biologics and drug encapsulation Read more
-
Microfluidics Article Reviews A mRNA encapsulation platform integrating Fluigent’s FlowEZ Read more
-
Microfluidics Article Reviews Microfluidic technology for engineered nanoparticles in nanomedicine Read more
References
1. Kossaï, M., Leary, A., Scoazec, J.-Y. & Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 85, 41–49 (2018).
2. Torre, L. A. et al. Ovarian Cancer Statistics, 2018. CA. Cancer J. Clin. 68, 284–296 (2018).
3. Anoikis mechanism and its involvement in metastasis adapted to BioRender template
4. Qu, Q., Zeng, F., Liu, X., Wang, Q. J. & Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 7, e2226–e2226 (2016).
5. Lesoin, L. Formation de liposomes par un procédé innovant utilisant les fluides supercritiques.
6. Internalization of liposome by BioRender adapted to Sercombe, L. et al. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 6, 286 (2015).