Creating a Microfluidic Cancer-on-Chip Platform using Fluigent’s High Throughput Cell Perfusion Pack
Erasmus MC, a prominent international academic hospital, collaborates with Bi/ond, a biotechnology company, to develop a Cancer-on-a-Chip platform. This innovative device is capable of sustaining cell viability, proliferation, and tissue structure in breast cancer patient-derived xenograft (PDX) slices for a minimum of 14 days.
The platform's ease of use and compact size make it a versatile tool for various ex vivo studies, including functional genomics, drug screening, and personalized medicine research.
Introduction
Erasmus MC is a leading international academic hospital at the forefront of the medical field. Its staff, volunteers, and students collaborate to provide healthcare for patients with complex disorders, rare conditions, and urgent medical needs. Recognized as a world-class scientific research organization, Erasmus MC strives to improve their understanding of diseases and disorders, working towards prediction, treatment, and prevention. The institution’s guiding principle revolves around the integration of biomedical research, clinical research, and health sciences to form a comprehensive approach to the advancement of healthcare.
Bi/ond, an innovative and international biotechnology company based in the Netherlands, was created with the primary aim of harnessing the potential of microchips to drive biological innovation. Working in close partnership with biologists, the company focuses on the creation of reproducible and accurate biological models that help develop inclusive and precise cures for medical issues. By drawing on its expertise in microelectronics and its in-depth knowledge of biological solutions, Bi/ond succeeds in bridging the gap between the fields of biology and engineering.
These two entities have combined their knowledge and technologies to develop an innovative Cancer-on-Chip (CoC) platform for assessing response to treatments using Fluigent’s high throughput cell perfusion pack.
Learn more about the Erasmus MC Cancer Institute
Learn more about Bi/ond

Testimonial

“We started out as a novice to the field of organ-on-chip cultures. Fluigent was very helpful at this stage to get started and keep the system up and running. They were always there to answer questions. Therefore, the precision pumping system was a thing we did not have to worry about, and we could concentrate on our own expertise, the biological materials in the culture device.”
Dr. D.C. (Dik) van Gent PhD
Universitair Hoofd Docent – Molecular Genetics
Why develop a Cancer-on-Chip (CoC) device to predict drug response?
Overcome the challenges in cancer treatments
Searching for personalized therapy treatment for individual patients is a challenging process. The crux of the problem lies in precisely defining the optimal treatment regimen for each individual. Although many molecular biomarker-based treatment strategies have been employed in cancer therapy, their ability to reliably predict individual responses to chemotherapy remains limited in most cases. This means there is a pressing need for ex vivo bioassays capable of effectively predicting a patient’s response to specific treatments, thus facilitating the selection of the most appropriate and effective therapeutic approach to optimize life expectancy and quality of life.
Historically, cancer cell lines and animal models have played a key role in assessing the efficacy of chemotherapies. However, when it comes to predicting tumor sensitivity in individual patients, these preclinical models fall short. Their main utility lies in studying the general characteristics of specific tumor types or stages of disease, which fails to consider the heterogeneity found in cancer and undermines their predictive power for responses to individualized treatment. These models are also time-consuming to set up and use, which limits their usefulness.
One potential solution lies in the direct assessment of drug responses using patient tumor tissue slice cultures. These ex-vivo cultures maintain the entire tumor microenvironment, including immune cells, and preserve the original tissue’s architecture. However, the development of long-term ex vivo culture systems, particularly those lasting more than 7 days, remains a major hurdle. The difficulty lies in managing mechanical stress on tissue slices, which can lead to the disruption of tissue integrity and non-physiological behavior, as well as in ensuring optimal culture conditions.
Consequently, it is crucial to invest in the development of more physiologically relevant ex vivo tissue slice culture systems, such as a cancer-on-chip platform, which enables the prolonged culture of tumor slices under precisely controlled conditions. Such advances could revolutionize personalized medicine and dramatically improve cancer treatment by enabling more precise and effective therapies, tailored to each patient’s specific needs.
The development of the organ-on-chip platform, an innovative technology
Despite considerable progress in computational and in vitro biology and toxicology over the past two decades, the failure rate of experimental drugs in clinical trials remains high, with over 80% of drugs failing to reach the market. Of these failures, 60% are attributed to lack of efficacy and 30% to toxicity. This situation has given rise to growing concerns about rising costs, wasted time, and ethical problems associated with animal experimentation, which often proves inadequate for predicting human reactions in a clinical context.
In addition, traditional live-cell experiments using cells grown on 2D substrates coated with serum or extracellular matrix molecules present limitations. Although they promote cell proliferation, they often fail to reproduce tissue-specific functions. Human organs, with their diverse functions, rely heavily on complex interactions between specialized cell types at well-defined interfaces, arranged in complex geometries and responding to specific microenvironments. Due to these issues, there is an urgent need for new modeling and testing platforms capable of better predicting human responses.
Organ-on-a-chip (OoC) technology represents a significant advancement in drug discovery and development, offering new tools for disease modeling and characterization, as well as potentially more accurate methods for assessing the toxicity and efficacy of new compounds and therapies. The organ-on-a-chip concept involves reproducing the functions of human physiology or disease at organ level in microfluidic chips, using different cell types.
Microfluidics plays a crucial role in enabling precise control of the cellular microenvironment, presenting cells with mechanical and biochemical signals in a more physiologically relevant context. Working with liquid volumes in the microliter range, these models enable dynamic scaling and interaction between cells. In addition, microfluidic chips can use geometries and structures to mimic physiological length scales with concentration gradients and mechanical forces generated by fluid flow, thus recreating the in vivo microenvironment faced by cells. This biomimetic approach, involved in the cancer-on-chip platform used in this study, overcomes many of the limitations encountered with conventional tissue culture models. [1]
How to combine OOAC and therapy assessments
The CoC platform provides continuous media perfusion, nutrient supply, waste removal, and the ability to collect samples for analysis. The aim of this platform is to develop a reproducible culture system for assessing the sensitivity of (breast and prostate) tumors to chemotherapy, using living material that closely resembles the original tumor and allows long-term culture without significant changes in viability or tissue characteristics. In addition, the system must enable a direct assessment of response to treatment through microscopic imaging and analysis based on fluid sampling.
In this article, the Cancer-on-a-Chip (CoC) microfluidic platform described uses an 6-well plate with silicon-based microfluidic chips. These chips offer greater flexibility than glass-based culture systems, as they allow for the easy integration of sensors for pH detection, metabolite screening, and oxygen sensing. In addition, the silicon-based design enables parallelization, taking advantage of semiconductor technology to improve scalability, reproducibility, and cost-effective large-scale production.
One notable application of this new Cancer-on-a-chip platform is personalized medicine. It facilitates the in vitro culture of tumor tissue slices under precisely controlled conditions, enabling the prediction of in vivo tumor responses to therapy in individual patients. The platform has been successfully used to grow tumor slices, including patient-derived xenografts (PDX), and faithfully mimicked tumor cells.
Use of the high throughput cell perfusion pack in the Coc platform to predict drug responses
Mimicking the in vivo cancer tissue on a microfluidic chip
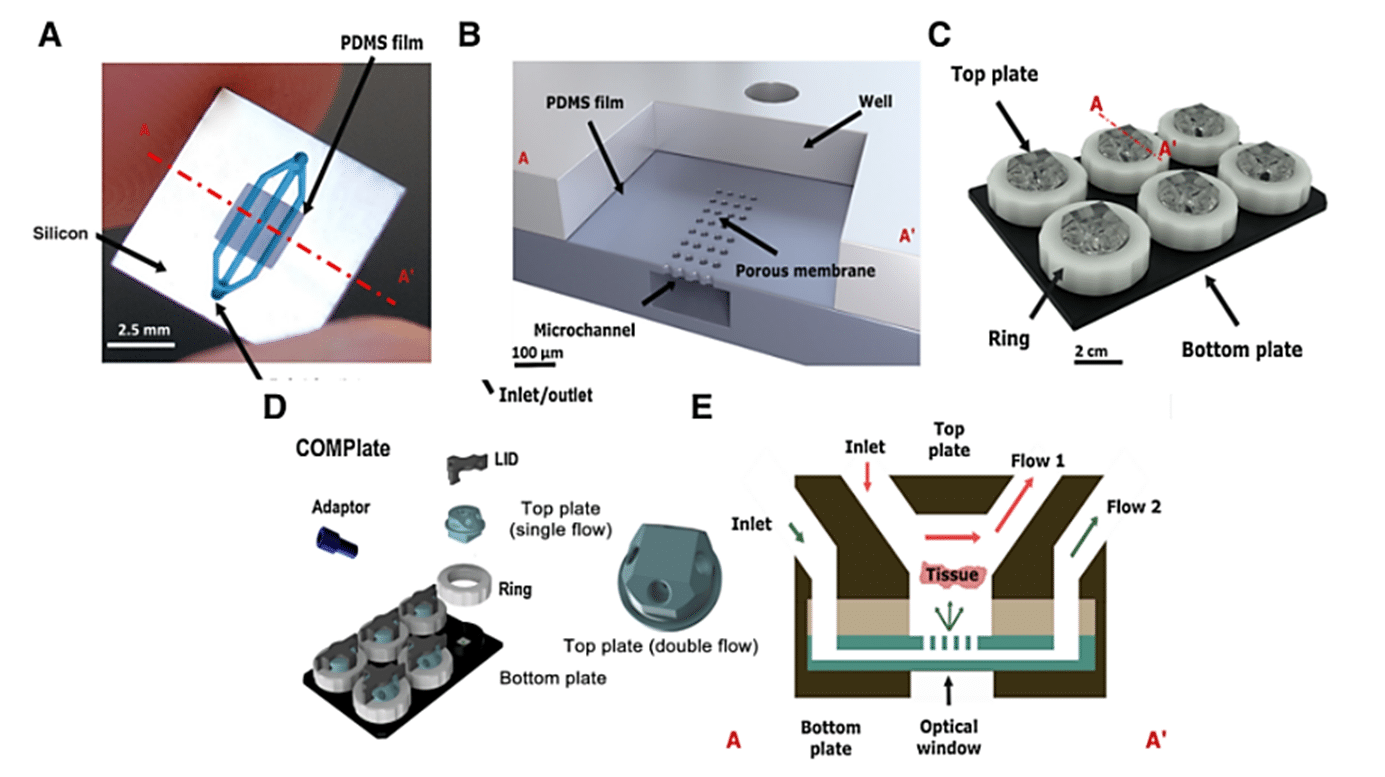
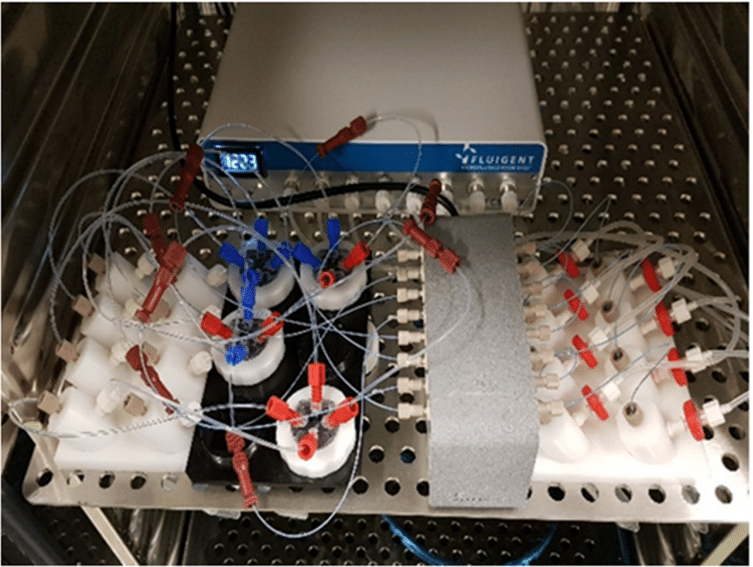
In this study, the microfluidic chips from Bi/ond are made of polydimethylsiloxane (PDMS) film with embedded microfluidic channels, supported by a silicon (Si) frame. The chips’ top plates serve as an interface connecting the inlet and outlet, enabling media diffusion through the tissue-supporting membrane. The chip features four microfluidic fittings for the external pumping systems. The bottom part of the chip is designed to ensure compatibility with microscopes and oxygenation, achieved through PDMS window openings under the chips.
The microfluidic chips containing tumor tissue slices are housed in a ComPLATETM. The ComPLATETM, designed by Bi/ond, is a smart, compact, and reusable well-plate specifically tailored for cultivating complex tissues. The plate is comprised of a black 6-wells bottom plate, a transparent top plate to cover the chip, and a white fixation ring. This design helps create independent cultivation and the analysis of individual tumor slices.
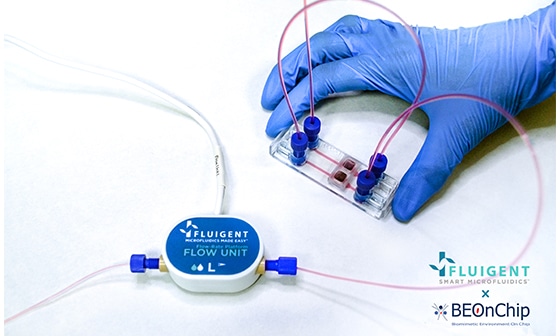
The top plate of the ComPLATETM provides the option for single or double flow of media. Each well has sufficient space to accommodate the tissue slice’s growth over time. The microchannel and top interface facilitate constant perfusion and nutrient replenishment, enabling maintenance of the tissue slices while removing waste products. Furthermore, oxygenation of the tissue slices is enhanced through a gas exchange via the PDMS layer of the optical window.To monitor fluid flow rates inside each well throughout the culture period, the entire Cancer-on-a-Chip platform is connected to a Fluigent Microfluidic Flow Control System.
A, Top view of the microfluidic chip illustrating its components: the PDMS film in which the microfluidics are embedded, and the silicon frame, which includes the inlet and outlet to the channels in the film. B, Vertical cross-section of the microfluidic chip. C, Representation of the CoC platform. D, ComPLATETM device. E, Cross-section of CoC illustrating the diffusion and perfusion toward the tissue slice.
Using Fluigent technology to ensure a high throughput cell perfusion
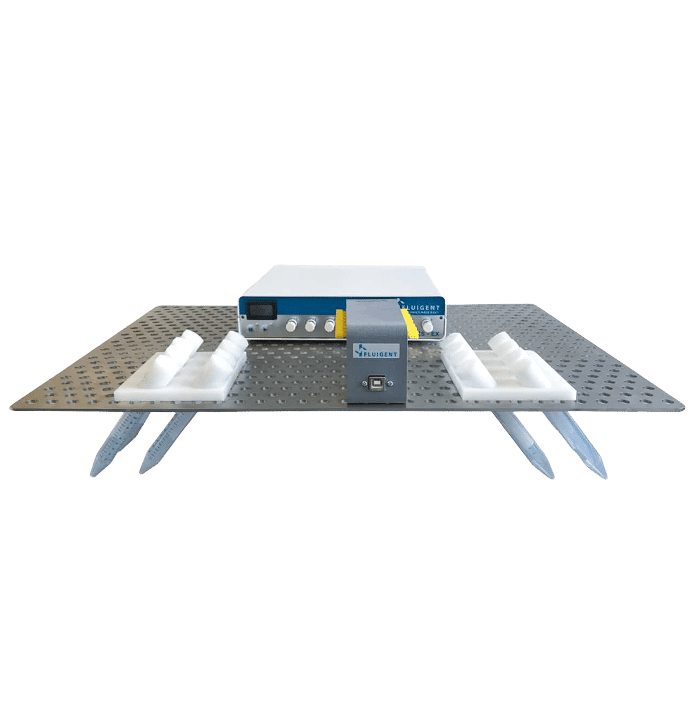
The Fluigent Cell Perfusion Pack is specially designed for high-throughput experiments. It has been carefully optimized for maximum efficiency in multiple-chip perfusions, enabling the simultaneous growth of multiple organ models in one incubator, which is ideal for the cancer-on-a-chip platform. The package includes a compact 8-channel pressure controller, a flow platform, and reservoir support that can be easily integrated into an incubator track. The user-friendly interface makes it easy to set up and operate the system, creating the proper physiological conditions for effortless long-term experiments. The system is highly reproducible and scalable.
To achieve continuous perfusion, an FLPG Plus pumping system was used as the pressure source. The flow rate was then maintained using the MFCSTM-EZ pressure-controlled microfluidic flow control system. Flow sensors (FLOW UNIT-S) were used to monitor flow throughout the culture using Fluigent software. A precise inlet flow rate of 5 µl/minute was used to perfuse PDX tissue slices through the chip’s upper and lower channels. CoC tissue was cultured under optimal conditions in a humidified atmosphere with 5% CO2 at 37°C, and the culture medium was renewed every 3 days for up to 2 weeks.
Comparison with an ex-vivo model
One of the aims of this study is to compare this innovative cancer-on-chip platform with a more traditional ex-vitro model. This traditional model consists of a cell culture in a 3mL customized culture with medium 6-well standard plates on an orbital shaker at 60 rpm. This will demonstrate the added value of using a platform based on Organ-On-a-Chip technology compared to more conventional methods. This will be made possible by carrying out various tests and comparing the data obtained by these two methods. These tests consist of studying the treatment response of tumors, long-term tumor tissue slice culture, and gene expression analysis.
Partial results
Treatment response of tumors
To assess the validity of the platform, a crucial question is whether in vivo treatment responses can be predicted by treatment responses. This validation was carried out using the cisplatin treatment on PDX breast cancer tumors (cisplatin-sensitive and cisplatin-resistant) with three biological replicates each.
To verify that the platform retained the essential features of tumor-associated cell morphology and proliferative capacity, untreated tumor slices at day 0 and day 7 were evaluated.
The effect of cisplatin treatment on cell proliferation and death was assessed in tissue slices grown under normal ex vivo conditions and in the CoC device. Cisplatin-sensitive tumor slices in the platform showed a significant increase in apoptotic cells and a notable decrease in replicating cells upon cisplatin treatment. In contrast, cisplatin-resistant PDX tissue slices showed no significant changes in either signal compared with untreated controls. [2]
The response to cisplatin treatment observed in the Cancer-on-a-Chip platform correlates with known tumor responses in in vivo and ex vivo cultures, suggesting its reliability for drug response analysis. Interestingly, breast PDXs cultured in the CoC platform showed a more robust response to cisplatin treatment compared with the ex vivo culture method, indicating better drug delivery in tumor slices with the platform.
To evaluate the performance of the Cancer-on-chip device in another tumor type, the PC82 androgen-dependent prostate tumor was used under the same conditions. The results led to the same conclusion as the breast tumor slices. [3]
In conclusion, CoC cultures accurately reproduced tumor responses to two different treatments (prostate and breast) in breast and prostate tumor models known to be sensitive in vivo.
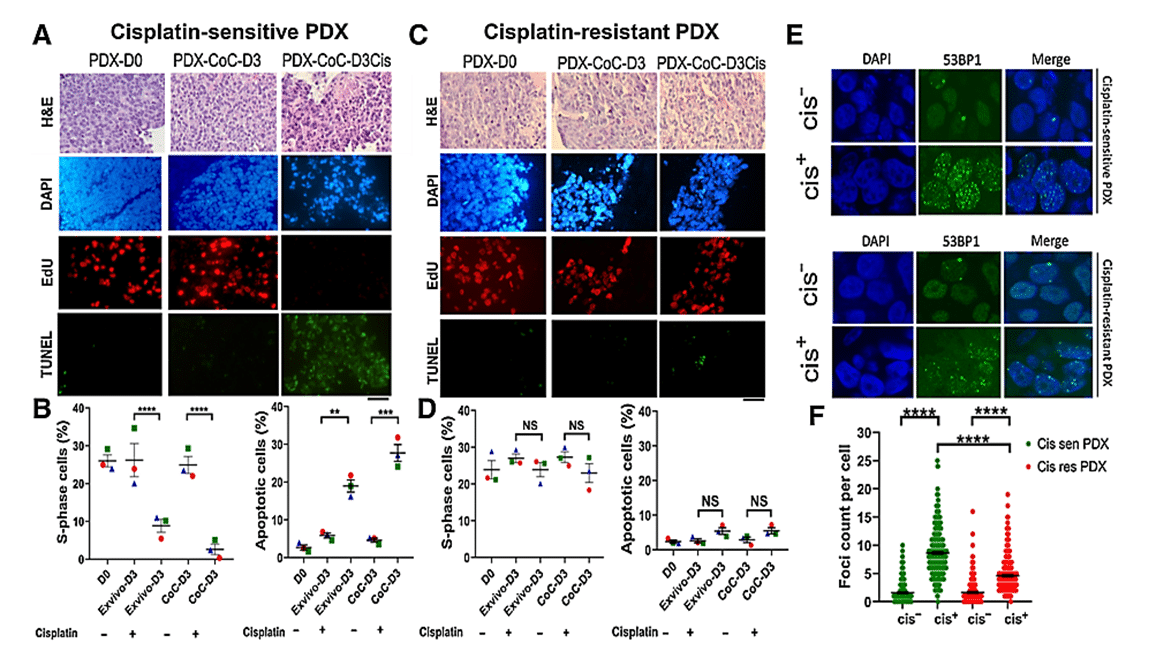
Figure 3: Prediction of therapy response using cisplatin-sensitive and -resistant PDX in ex vivo and CoC platforms. A, Representative EdU (proliferation) and TUNEL (apoptose) staining of cisplatin-sensitive breast PDX. B, Quantification of the fraction of EdU-positive and TUNEL-positive cells showing breast PDXs were sensitive to cisplatin. C, Representative EdU and TUNEL staining of cisplatin resistant breast PDX. D, Quantification of the fraction of EdU-positive and TUNEL-positive cells showing breast PDXs were insensitive to cisplatin therapy, thereby validating the application of CoC for therapy response for patient tumors. E, Analysis of DNA damage response in cisplatin-sensitive and -resistant PDX treated with cisplatin. Cisplatin treatment induced more double-strand breaks in cisplatin-sensitive PDX than in cisplatin-resistant PDX. F, Scatter plot showing 53BP1 foci count per cell in cisplatin-sensitive and -resistant PDX.
Long-term tumor tissue slice culture
Longer culture times are essential for studying therapeutic responses that require longer incubation periods-more than one week-such as the development of therapeutic resistance or clonal outgrowth. The main limitation observed in ex vivo culture is the preservation of optimal proliferative capacity and tissue architecture.
To address this, a study was carried out using breast tumor slices from five independent patient-derived xenografts. These slices were cultured for 14 days in the Cancer-on-a-Chip platform and, in parallel, in the ex vivo 6-well plate. After 7 days of culture, similar rates of cell proliferation in the CoC device compared with day 0 were observed, but slightly slower proliferation in the ex vivo condition. TUNEL staining revealed a slight increase in cell death in the ex vivo system compared with the CoC platform at day 7.
Notably, these differences became more pronounced in extended 14-day cultures. The platform showed better preservation of tumor tissue architecture and cell proliferation than the ex vivo culture system. In contrast, the ex vivo condition showed a significant decrease in proliferation at day 7 compared with PDX tumors at day 0, whereas no significant difference was observed between day 7 and day 0 for the platform, indicating slightly slower cell proliferation in ex vivo culture compared with CoC.
These results underline the superiority of the Cancer-on-chip device for prolonged culture times (beyond 7 days) of tumor tissue slices compared to the ex vivo system (better preservation of cell integrity and cell proliferation).
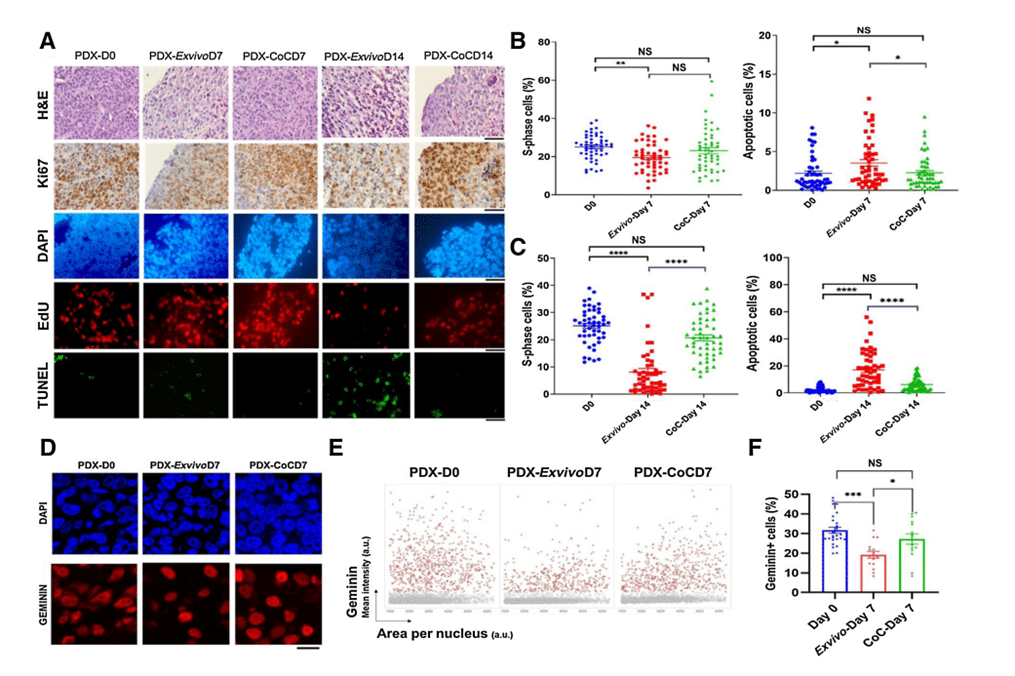
Figure 4: Breast PDX tumor tissue slices cultured in ex vivo condition and in CoC device for up to 14 days. A, Quantification of the fraction of EdU-positive and TUNEL-positive cells for 5 breast PDX tissue slices cultured for up to day 7 (B) and day 14 (C). D, Representative image showing breast PDX tumors labeled with geminin (red nuclei) and DAPI (blue nuclei). E, QIBC analysis of three independent breast PDX tumors with more than 3,000 cells analyzed for each are shown in each condition. F, Quantification of geminin-positive cells showed CoC at day 7 had similar cell proliferation profile as in day 0 than ex vivo condition.
Gene expression analysis
To assess the impact of CoC culture on gene expression changes, analysis using RT-PCR and whole transcriptome sequencing was performed. Tumor-specific gene pathways in PDX breast tumors cultured ex vivo and in the Cancer-on-a-Chip platform were examined. Surprisingly, there were no statistically significant changes in these pathways, suggesting minimal alterations in tumor growth characteristics under both conditions. Next, whole transcriptome sequencing of PDX breast tumors was performed. Genes were identified as differentially expressed on day 7 ex vivo and on days 7 and 14 under CoC culture conditions. 150 human genes are differentially expressed in day 7 ex vivo tumor slices, far more than the 30 human genes differentially expressed in day 7 CoC and the 14 human genes in day 14 CoC.
To understand the reasons for the differences observed, various tests studying cell cycle progression and apoptosis were carried out. They led to the conclusion that ex vivo culture conditions induced greater immune activation and DNA damage after 7 days, making the CoC system a more accurate representation of the original tumor and the preferred choice for studying responses to therapies.
Conclusion
The researchers developed a microfluidic CoC platform capable of maintaining cell viability, proliferation and tissue structure in breast cancer PDX slices for at least 14 days. This platform successfully predicted responses to cisplatin therapy for breast cancer and antiandrogen therapy for PDX prostate cancer tumor slices. To fully establish its potential as an in vitro diagnostic test for therapy selection, it will require clinical validation using biopsies from patients receiving the same chemotherapy.Although the current study has focused on PDX models of breast and prostate cancer, this Cancer-on-a-Chip platform also holds promise for other solid tumors. Its ease of use and small footprint make it a versatile tool for ex vivo studies, including functional genomics, drug screening and personalized medicine research.
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics Pressure-Controlled Microfluidics in Organ-On-A-Chip Research Read more
-
Microfluidic Application Notes Long-term fluid recirculation system for Organ-on-a-Chip applications Read more
-
Microfluidics Case Studies CNRS/UTC: study of a liver-on-a-chip model Read more
-
Interviews & Testimonials Panel Discussion & Interviews – Microfluidics & Organ-On-Chips Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
Related Webinars
Related products
References
[1] Microfluidic white paper – A guide to Organs-on-Chips technology, Fluigent
[2] Naipal KA, Verkaik NS, Sanchez H, van Deurzen CH, den Bakker MA, Hoeijmakers JH, et al. Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer 2016;16:78
[3] van Weerden WM, van Steenbrugge GJ, van Kreuningen A, Moerings EP, de Jong FH, Schr€oder FH. Assessment of the critical level of androgen for growth response of transplantable human prostatic carcinoma (PC-82) in nude mice. J Urol 1991;145:631–4