Automated immunolabeling to perform highly multiplexed tissue imaging with ARIA
The Chu Lab is a recently established group at University College Institute of Ophthalmology in partnership with Moorfields Eye Hospital led by Dr. Colin Chu. It is dedicated to ocular immunology, immune cell imaging, spatial biology and gene therapy.
Understanding cell-cell interactions and spatial relationships in healthy or diseased organs represents a technical challenge. Single-cell genomics is powerful but lacks spatial context. In this study, automated immunolabeling has been applied to obtain a versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues.

“As a clinician and immunologist with an interest in imaging, I was involved in developing a new technique for multiplexed immunohistochemistry. We wanted to automate the process by adding a microfluidic solution, but I had no experience with this technology. The ARIA system fit our needs and was incredibly simple and intuitive to use. The software is well-designed and readily interfaced with our microscope. Fluigent provided support throughout setup and transformed what seemed a large undertaking, into an incredibly simple task.”
Colin Chu, University College London
Introduction
Dr. Colin Chu has recently established his own lab at University College London (UCL) Institute of Ophthalmology in partnership with Moorfields Eye Hospital. As an ophthalmologist specialized in ocular immunology, his research is focused on using in vivo and ex vivo imaging to better understand the causes of blinding diseases.
While he was a visiting fellow in the lab of Dr Ron Germain at the National Institute of Health (NIH) in Maryland, USA, Dr Chu helped establish an automated highly multiplexed immunohistochemistry approach with his colleague Dr Andrea Radtke. This work was published in Nature Protocols in February 2022.
It uses an automated immunolabeling technique called IBEX, to achieve over 30 distinct antibody markers on the same section of tissue. Previously this had only been performed manually, limiting the throughput, speed and accessibility of the technique. Working together with Fluigent to connect an ARIA microfluidics system to a Leica THUNDER microscope, the two systems were able to interface to deliver automated staining and image acquisition.
Dr. Chu is now setting up the system at UCL to apply the automated immunomarking technique, IBEX, to the retina in mice, zebrafish and human post-mortem tissue. In addition to multiplexed tissue imaging with immunofluorescence, his research topics include ocular gene therapy and in vivo immune cell imaging using adaptive optics, optical coherence tomography (OCT) and fluorescence microscopy to better understand ocular inflammation.
How to perform automated immunofluorescence
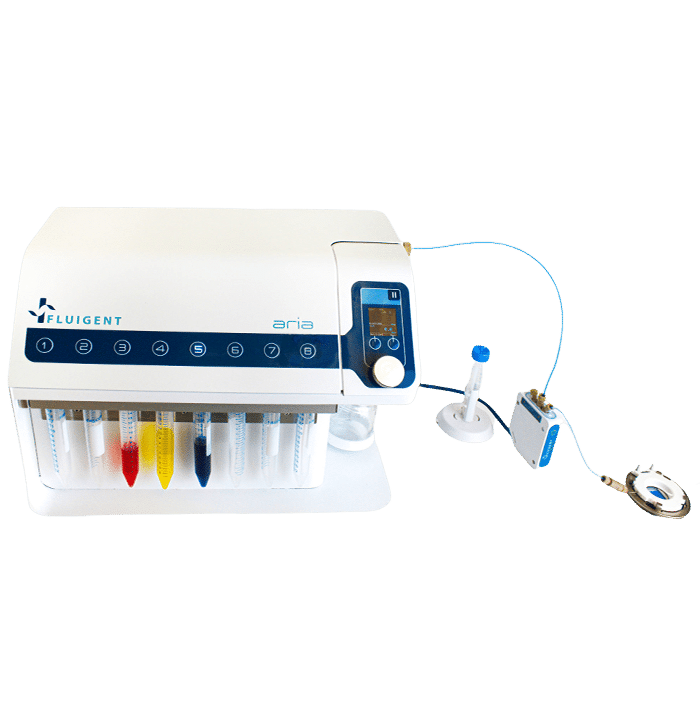
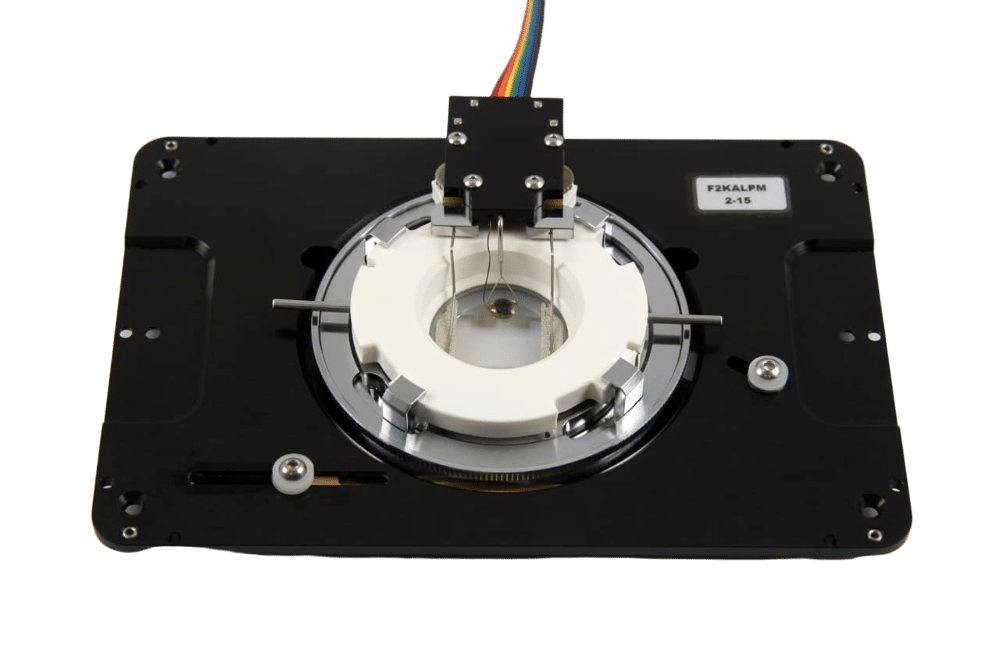
Fluigent instrument ARIA coupled to a widefield microscope to perform automated multiplexed tissue imaging
High content imaging of tissue samples was performed using an automated immunolabeling method, consisting of iterative cycles of antibody labelling, imaging, and fluorophore bleaching using lithium borohydride (LiBH4). Image processing and registration were done using the SimpleITK open-source software.
To perform an automated IBEX study, the complete setup comprised several pieces of equipment:
- a pressure source (here our FLPG)
- a single output sequential perfusion system ARIA
- a closed bath imaging chamber containing tissue sections and stage heater from Warner Instruments
- a THUNDER microscope from Leica
Using ARIA software, a method containing all the fluid injection and image acquisition steps was created. Once loaded, the sequence can be run automatically. The automated immunomarking method relies on TTL signals sent and received by ARIA, enabling a cycle of image acquisition to be launched at the end of an injection step. The software interfaced with the Leica THUNDER microscope.
Once an image was acquired, the next fluid injection step started, to bleach the previous round, commence antibody staining with the next panel, then trigger microscope acquisition again. Ultimately, the full sequence of steps gave rise to high-quality, multiplexed protein level data of over 30 markers.
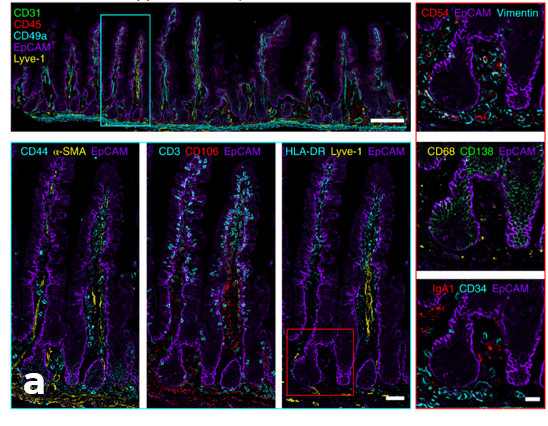
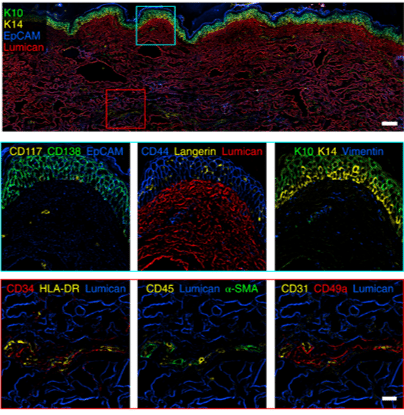
Fixed human tissues including mesenteric lymph node, small intestine and skin were profiled (Figure 1, 2). These tissues are usually very challenging to image due to their delicate structure and high background autofluorescence.
As stated by the authors, the original IBEX method was limited by the requirement of manual execution (manual pipetting for antibody staining and fluorophore inactivation). Here, ARIA provides an affordable automated solution to access high-content imaging as part of the spatial biology revolution.
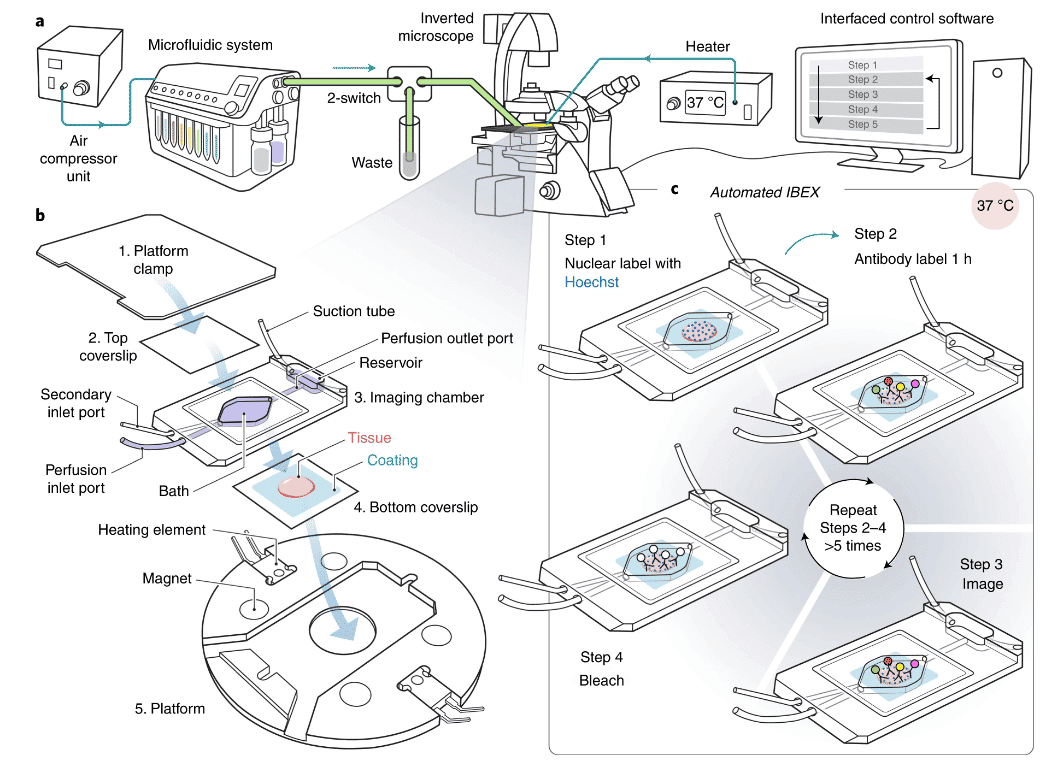
IBEX automated immunomarking method: Experimental procedure
a) The automated IBEX protocol uses our compact microfluidics system ARIA to deliver multiple solutions to an imaging chamber placed on an inverted microscope stage. Fluids are moved through the system with compressed air and delivered to the imaging chamber based on protocols defined by the user. The inverted microscope and ARIA device communicate via TTL signals using precise timing established by the user with the Aria control software.
b) Samples are sectioned onto coated coverslips and assembled into a closed bath imaging chamber. Following assembly, the imaging chamber is secured to a magnetic platform and mounted onto the microscope stage.
c) Automated IBEX consists of: (1) nuclear labeling with Hoechst, (2) antibody labeling for 1 h at 37 °C using a heated microscope stage, (3) imaging ROIs, (4) bleaching with LiBH4, and (5) repeating Steps 2–4 until the desired number of parameters is achieved, typically 12 h for a six-cycle, 25-plex experiment.
Partial Results
Radtke AJ. *, Colin CJ*. et al, have established a high throughput automated immunolabeling protocol recently published in Nature Protocols based on the automation power of our Fluigent ARIA instrument coupled to an image alignment open-source software package.
This powerful approach relies on iterative staining and bleaching method to perform high-resolution imaging of more than 65 parameters. This Iterative Bleaching Extends Multiplexing protocol (IBEX) represents a robust and reproducible method to perform deep phenotyping and spatial analysis of cells in complex tissues (healthy organ, infected organs, tumor microenvironment).
Examples of Images obtained using automated IBEX method in human tissues
Conclusion
In this paper, the authors have demonstrated the automation power of our sequential perfusion system ARIA together with its ability to be synchronized with a widefield (or other) microscope to perform automated and multiplexed antibody labelling. ARIA can send and receive TTL signals to launch an image acquisition cycle and resume the perfusion protocol once the imaging cycle has been completed. Other applications such as DNA-paint, OligoSTORM, dose/response studies, automated multiplex immunofluorescence experiments or dynamic pulse-chase experiments can be automated using ARIA. .
- Chu Lab website: https://colinchulab.com/
- UCL Chu Lab website: https://iris.ucl.ac.uk/iris/browse/profile?upi=CHUXX55
- General UCL website: https://www.ucl.ac.uk/
Additional information about Aria
Aria’s technology, enabling automated sequential injection for cellular perfusion or timed injection protocol, is a new state-of-the-art technology that holds great promise for many cell biology and organ-on-chip cell culture projects. Incorporating Fluigent’s latest technologies, this instrument ensures perfusion with minimal shear stress and highly controlled flow. To find out more about this technology, we invite you to visit the product web page, or take a look at our Webinars.
Aria is the only instrument that can automate the delivery of up to 10 different solutions to a flow cell. It’s the perfect compromise between manual pipetting and an all-in-one system dedicated to one specific application. Any protocol with multiple solution delivery can be automated, saving the scientist both time and reducing variability between experiments compared to procedures that do not include perfusion systems. Users can integrate their own microscope, specific chip type and solution sets using the Aria Automated sequential injection system.
Automate Cellular Studies with Aria
Unlock your path to success with Fluigent’s cutting-edge perfusion system, Aria. Say goodbye to the complexities of manual fluid delivery, and embrace a new era of seamless experimentation.
Automate with Aria:
When your experiments demand precision in timed delivery of reagents, probes, fluorophores, and more, all while reducing the need for constant operator intervention, look no further than Aria, our next-generation instrument for automating cellular perfusion studies.
Streamlined Efficiency:
In response to the feedback from our valued customers, we recognize the time and effort that multi-solution protocols often require, coupled with substantial operator involvement. Aria simplifies your research by offering swift experimental setup, user-friendly operation, and the ability to automate even multi-day protocols. Additionally, it seamlessly interfaces with various microscopes, further enhancing reagent delivery and imaging.
Your Solution for Lab Automation:
Aria is the ultimate solution to streamline your laboratory work. Its high flexibility allows adaptation to any perfusion chamber and protocol, thanks to its intuitive software.
Elevating Live-Cell Imaging:
Beyond its versatility, Aria serves as a powerful tool for automating complex protocols, particularly in the realm of live-cell imaging. Whether it’s immunostaining, omics applications, or radiometric imaging, Aria is your partner in simplifying intricate experiments.
Automated Fluid Delivery for Cell and Tissue Imaging
If you’re looking to save time, enhance precision, and achieve reproducibility in your immunofluorescence assay or any other assay that involves injecting multiple solutions into your samples, our webinar is a must-attend event. Discover how to seamlessly integrate a flow chamber or microfluidic chip with our automated fluid delivery device, ARIA. With this setup, you can deliver up to 10 different solutions sequentially and autonomously. Additionally, ARIA can be synchronized with any microscope to initiate an image acquisition cycle and resume the perfusion protocol once the imaging cycle is complete.
This all-in-one workflow streamlines the process of injecting/incubating with reagents and acquiring images, making it particularly well-suited for complex cell and tissue imaging. During the webinar, we will showcase various applications, including multiplexed tissue imaging, DNA-PAINT, seqFISH, cell capture, and staining.