A quick and efficient double encapsulation method for FACS-based droplet sorting
In this application note, we associate the double emulsion microfluidic platform with FACS sorting technology to sort out small droplets encapsulating fluorescent e-coli bacteria. Confining bacteria in small droplets is essential to perform single-cell analysis and to avoid fluorescence signal dispersion in bioassays based on protein or enzyme secretion.
Water-in-oil-in-water double emulsions efficiently play the role of both microbioreactor for efficient bacterial growth, and cellular activity and signal confinement, as the oil shell phase prevents fluorescence leakage.
Secoya developed and manufactured the RayDrop device used to perform this application note.

What is FACS sorting?
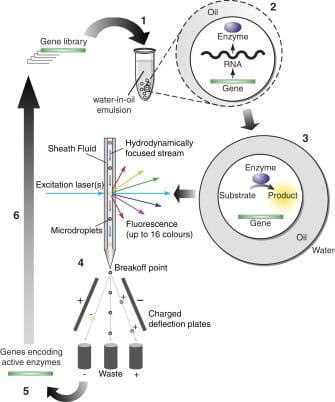
Analyzing cellular behavior at the single-cell level is important to life sciences as it enables a better understanding of cellular heterogeneity, which can be used for personalized medicine. FACS technology is a biology tool solution that offers an efficient selection of individual particles of interest among a complex population. FACS, or Fluorescence-Activated Cell Sorting, is based on the measurement of fluorescence signals at multiple wavelengths under laser excitation of cells flowing in line in a confined stream. The measured intensity gives information on the particle size, complexity, or specific phenotype, such as protein secretion. When the particle of interest is detected, an electric charge is applied, deflecting the cell towards a collecting tube.
Why is droplet sorting associated with droplet microfluidics?
Despite its potential for single-cell analysis and sorting, FACS sorting has two major limitations. First, it allows for single-cell analysis, but the analyzed sample usually is a bulk cell culture involving cellular interaction, preventing a real single-cell characterization (cells influence each other’s behavior). Second, FACS requires that the signal remains at the surface or inside the cells, which can be compatible with certain applications but often requires damaging cell treatment (i.e. fixation) and induces cellular death, preventing further analysis of the cell.
Droplet microfluidics removes these limitations. By confining single-cells inside droplets, droplet microfluidics allows for a full single-cell assay and ensures that the fluorescence signal stays in a closed area without using damaging chemicals. Droplet sorting using FACS technology recently emerged as a powerful tool to combine efficient sorting and single-cell analysis. Water-in-oil-in-water (W/O/W) double emulsions are of particular interest for droplet FACS sorting, since this application requires that the particles flow in an aqueous continuous phase [1].
In this application note, we encapsulate fluorescent bacteria in water-in-oil-in-water (W/O/W) double emulsions in one step using the FACS platform and perform droplet sorting using FACS technology to select only fluorescent particles.
This document was made in partnership with Delphine Lestrade, Head of the TWB (Toulouse White Biotechnology) cytometry platform, Sophie Lajus, researcher at the TBI (Toulouse Biotechnology Institute), and Sandra Pizzut-Serin and Sophie Bozonnet, from the ICEO-PICT platform.
Testimonial
As an expert in leading research and development (R&D) projects, TWB supports the industry via the development of innovative and sustainable solutions for the benefit of the planet and mankind.
TWB conducts R&D projects in the field of industrial biotechnology in collaboration with public laboratories and industrial players. They support the development of start-ups by offering them access to state-of-the-art scientific and technological environments and encourage the emergence of breakthrough innovations. By bringing together researchers, entrepreneurs, funders, institutions and industries, TWB integrates and leverages industry knowledge and simplifies the contractual relationship. This unique model accelerates the innovation process needed for the creation of an eco-responsible industry.

At TWB, the Cytometry Platform is responsible for developing and defining new protocols for high-throughput cytometry assays for the identification and characterization of microorganisms.
Delphine Lestrade, Head of the TWB (Toulouse White Biotechnology) cytometry platform
Webinar on double emulsion for FACS
Learn how to use our double emulsion platform to encapsulate bacteria in W/O/W DE and discover the DE sorting efficiency using FACS.
How to encapsulate bacteria in double emulsions
Materials
Reagents:
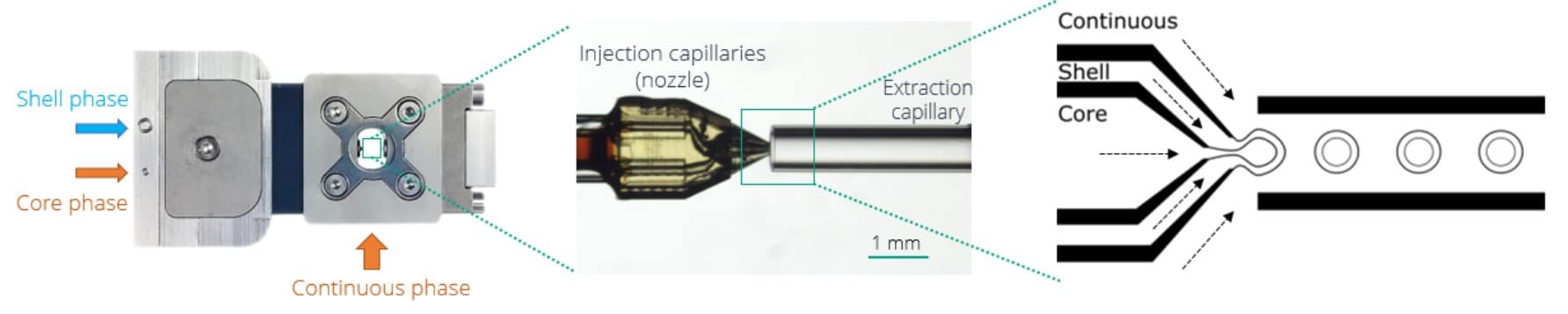
Making a double emulsion requires three different solutions: a core phase, a shell phase, and a continuous phase. The shell phase must be immiscible with the two other phases. Here, we add a fourth solution: the collection phase, in which we retrieve the emulsion for further droplet sorting. In addition, instead of having a simple core phase, we have a priming phase to start producing W/O/W DE, and an injection phase that contains biological materials (here bacteria in LB medium), that we introduce in the core fluid path using an injection loop.
Continuous phase: MilliQ water + 2% V/V Tween20
Shell phase: Dsurf 2% in HFE 7500 oil
Priming phase: MilliQ water for system priming and flow stabilization
Injection phase: LB culture medium with ~108/mL E.coli expressing the Green Fluorescent Protein (GFP) in the injection loop for bacterial encapsulation
Collecting phase: MilliQ water + 2% V/V Tween20 + 150mM NaCl
Products:
How to encapsulate e-coli in double emulsions
We start the experiment by filling the Raydrop with the continuous phase. When the microfluidic chip is full, we set the pressure on the shell phase’s flow controller and make simple droplets of the shell. Once the flow is stabilized, we progressively increase the core flowrate until a double emulsion is formed. The double emulsion‘s shell thickness can be tuned by playing with the core and shell flow-rate ratio. In addition, the droplet size can be controlled by increasing or decreasing the continuous flow rate. At this stage, a simple W/O/W double emulsion is made.
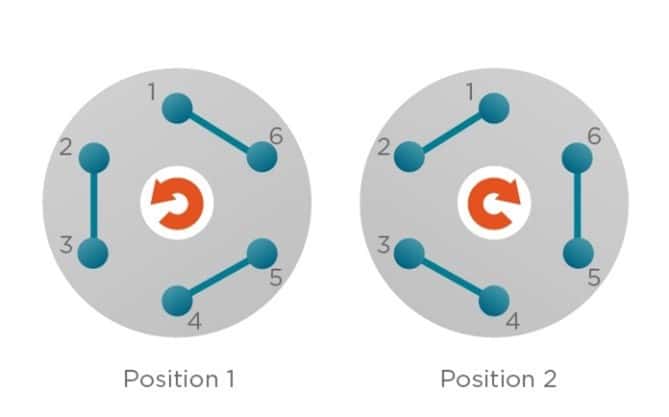
We then make sure that the L-switch is set on position 2 and fill the injection loop by injecting the bacterial culture in port 5. Then, we switch to position 1, and after a certain time depending on the core phase flowrate, encapsulation of e-coli begins. After some time (15 – 25 minutes depending on the flowrate), the encapsulation is over.
For more details about the encapsulation process, please refer to the application note.
- Expert Reviews: Basics of Microfluidics
10 Tips for Reliable Droplet Generation
Read more - Microfluidic Application Notes
What is the best method for Microencapsulation of Bacteria and Yeast in Small Double Emulsions?
Read more - Microfluidic Application Notes
Encapsulation of Cells In Small Double Emulsions
Read more - Expert Reviews: Basics of Microfluidics
Microfluidic Droplet Production Method
Read more
Microscopic validation
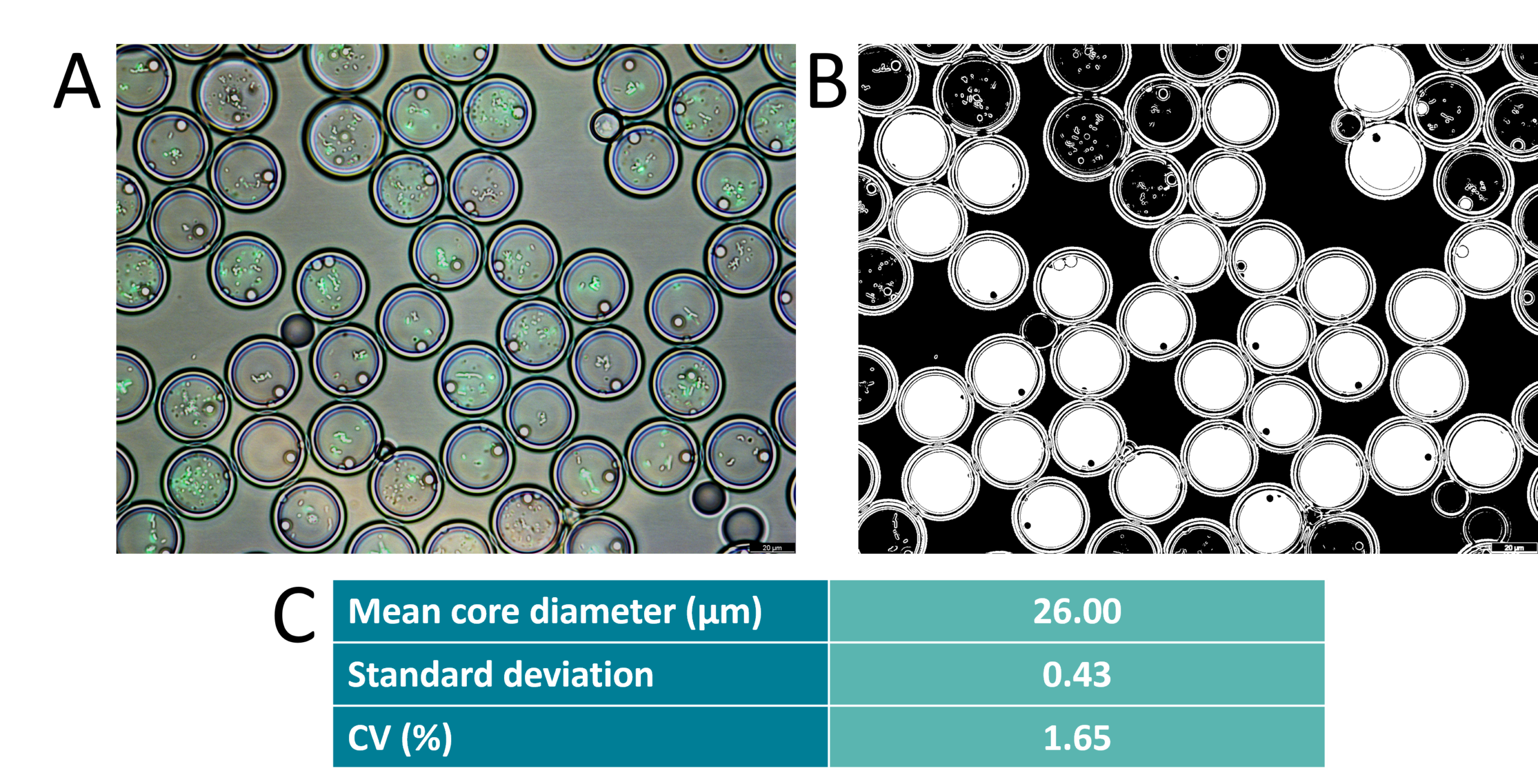
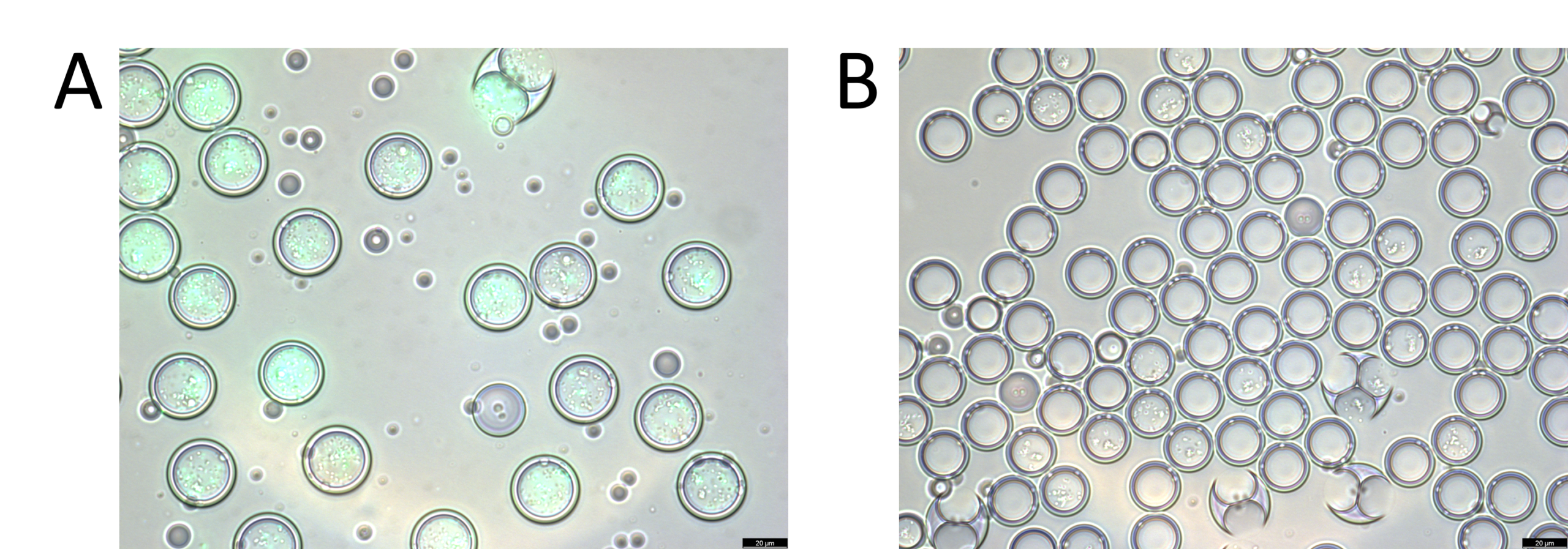
Before droplet sorting with FACS, particle size and monodispersity were assessed using microscopic imaging. The coefficient of variation of the core size was found to be 1.65% and the global droplet diameter has a coefficient of variation of 2%.
After microscopy validation, the double emulsions were passed through a Moflo Atrios FACS system for droplet sorting based on the fluorescence signal inside the drop. The fluorescent particles were collected in one tube and the non-fluorescent ones (empty or encapsulating non-fluorescent bacteria) in a second tube.
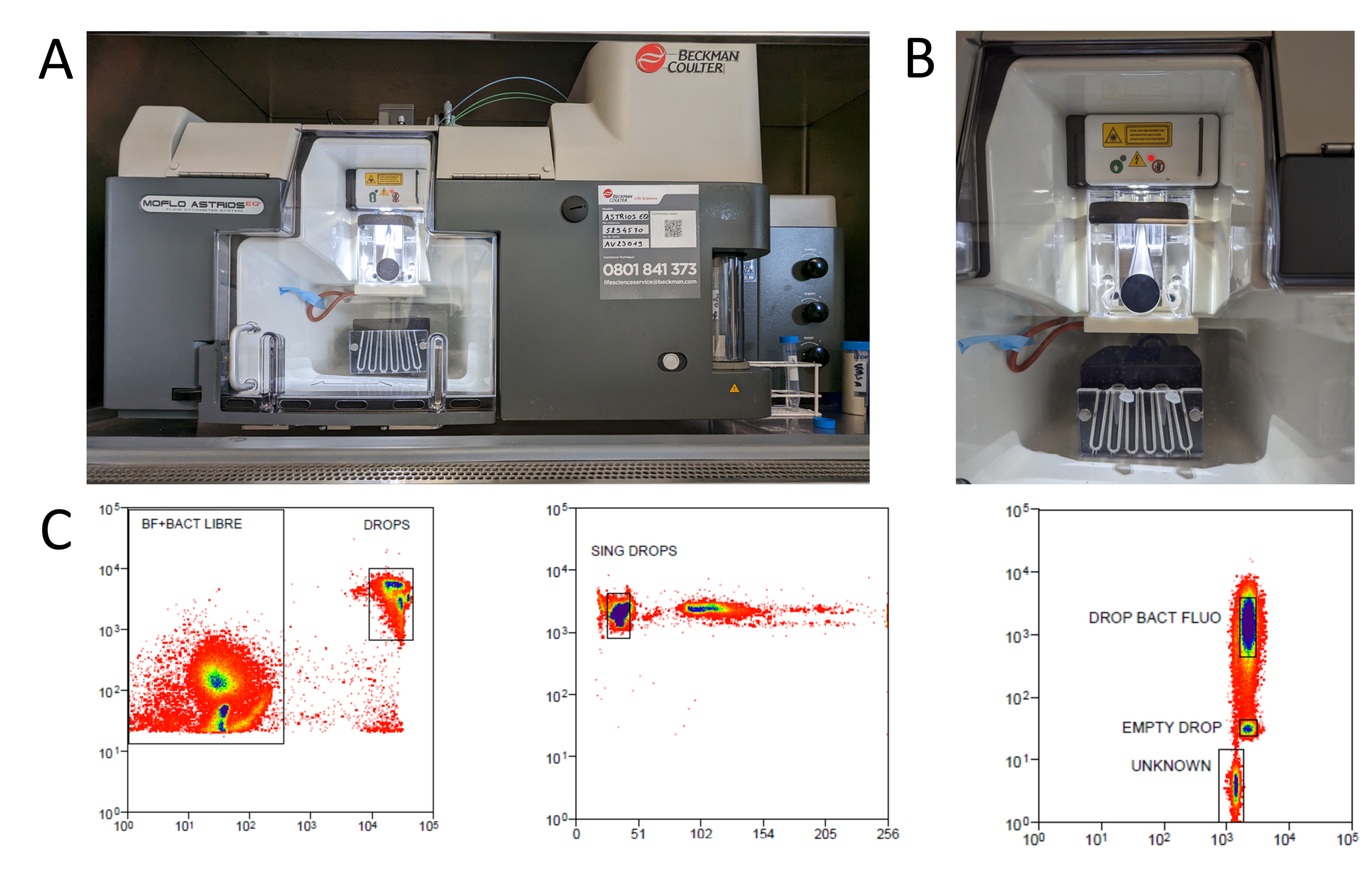
A microscopic validation was then performed on the content of both tubes. We notice that for the first vial, the droplets are all green florescent, whereas none of the droplets from tube 2 are. This confirms efficient droplet sorting. In addition, the fluorescence-activated cell sorting machine displayed a sorting efficiency of 75-95%.
Conclusion
FACS sorting was efficiently performed on double emulsions encapsulating Escherichia coli based on the measure of fluorescence inside the droplets. The W/O/W droplets generated with the platform showed good monodispersity, and the platform’s ability to generate high volumes of droplets in a short time with low biological sample consumption (200mL of double emulsion produced in <30min) was helpful for this application.
Related Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes What is the best method for Microencapsulation of Bacteria and Yeast in Small Double Emulsions? Read more
-
Microfluidic Application Notes Encapsulation of Cells In Small Double Emulsions Read more
-
Microfluidic Application Notes Double Emulsion Generation Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Droplet Production Method Read more
Webinar Recording
References
[1] K.K. Brower, C. Carswell-Crumpton, S. Klemm, B. Cruz, G. Kim, S.G.K. Calhoun, L. Nichols, P.M. Fordyce. Double emulsion flow cytometry with highthroughput single droplet isolation and nucleic acid recovery, LabChip, 2020, 20, 2062-2074
[2] E. Mastrobattista, V. Taly, E. Chanudet, P. Treacy, B. T. Kelly, A. D. Griffiths, Chemistry & Biology 2005, 12, 1291.
[3] DEWANDRE, Adrien, RIVERO-RODRIGUEZ, Javier, VITRY, Youen, SOBAC, Benjamin et SCHEID, Benoit, 2020. Microfluidic droplet generation based on non-embedded co-flow-focusing using 3D printed nozzle. Scientific Reports. 10 décembre 2020. Vol. 10, n° 1, pp. 21616. DOI 10.1038/s41598-020-77836-y.