Encapsulation of Cells In Small Double Emulsions
Droplet-based microfluidics, which produces large amounts of monodisperse droplets with precisely controlled size and tailored internal structure, has been proven to be a promising technique for cellular encapsulation of large, complex cells within highly monodisperse DE droplets small enough for high-throughput screening. In this application note, we describe the method for encapsulation of cells (Human Adult Peripheral Blood Mononuclear Cells (PBMC)) in double emulsions of 50 µm size using the cell encapsulation Platform.
Microfluidic droplet generation for encapsulation of cells
Microfluidic droplet generation is a powerful technique for encapsulation of cells or biological molecules within precisely controlled nL- to pL-volumes. Microfluidic droplets have been used for a wide variety of applications, including directed evolution of enzymes and proteins, digital PCR, large-scale gene assembly, cell culture, and, recently, single-cell genomic, epigenomic, and transcriptomic analyses (1,2).
Advantages and challenges of the method
The need for cell encapsulation methods and sorting devices that are safer, more effective, and simpler to use than current technologies has grown due to the exponential growth of novel techniques of cell analysis.
To perform it, double emulsions are ideally generated because, unlike single emulsions, they provide aqueous compartments as well as an aqueous carrier fluid, which makes the emulsion compatible with most flow cytometry and cell sorting systems.
For successful sorting, DE droplets must be significantly smaller (< 60 µm in diameter) than commercial cell sorters nozzles (typically 70–130 μm in diameter) while simultaneously large enough to encapsulate variants of interest within the inner core volume (4). Therefore, the possibility of developing a cellular encapsulation method in double emulsions small enough to be compatible with commercial cell sorters would be a major breakthrough in the field of biomedical research.
Typically, double emulsions are produced in batches by a two-step emulsification process, resulting in a highly polydisperse population with low encapsulation efficiency. Therefore, droplet generation using microfluidics is an alternative, as it offers maximum control over droplet generation (5).
A platform for high-throughput screening
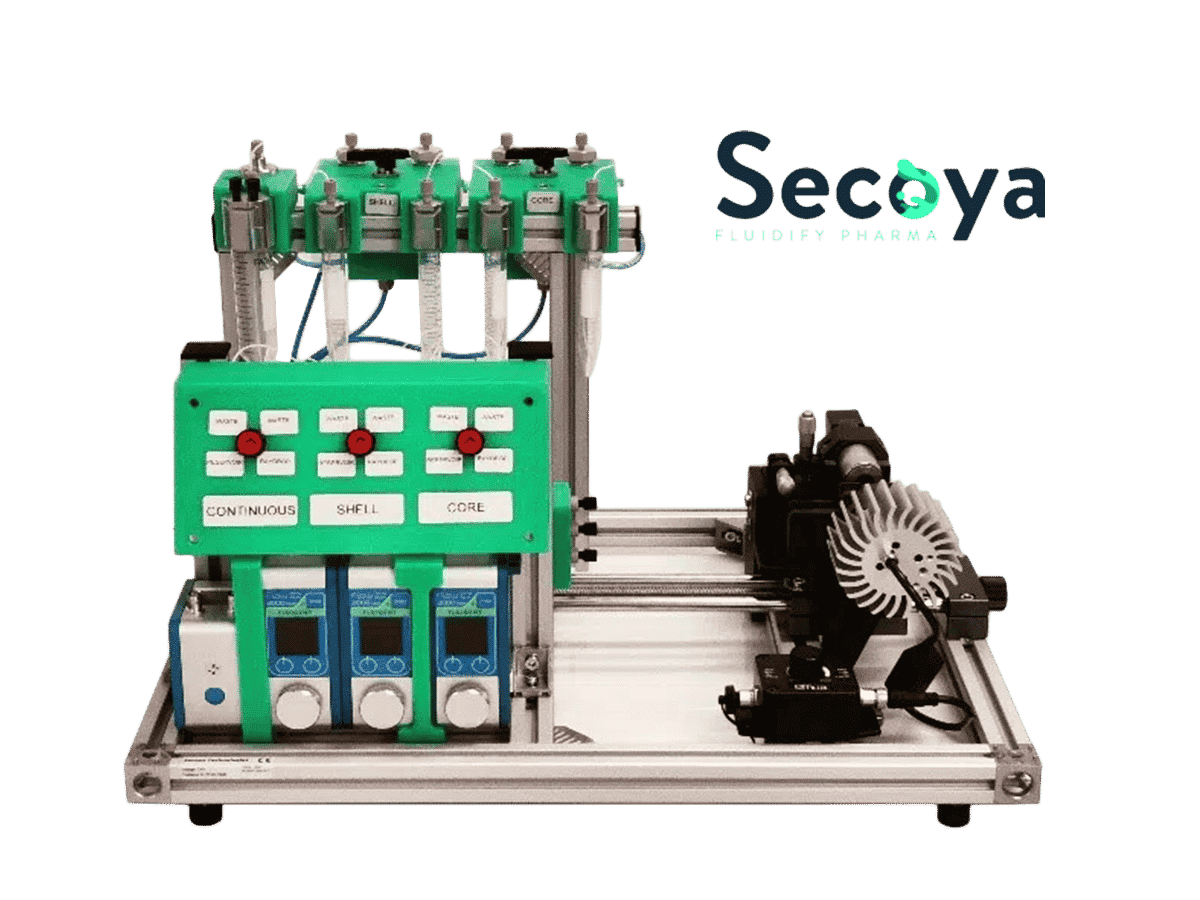
With the Cell Encapsulation Platform, developed and manufactured by Secoya, we demonstrate an easy-to-use and robust cellular encapsulation method for encapsulating large, complex cells within highly monodisperse DE droplets small enough (∼ 25µm to 60µm) for high-throughput cell screening/sorting. We demonstrate the capabilities of this method by encapsulating Human Adult Peripheral Blood Mononuclear Cells (PBMC) in highly monodisperse double emulsions of 50µm in size.
How to produce Small Double Emulsions?
Materials
While the shell phase is composed of dSurf (HFE7500 + 2% biocompatible surfactant), the core phase consists of water with 0.5% Fluorescein.
Finally, the continuous phase is composed of water with 2% Tween20.
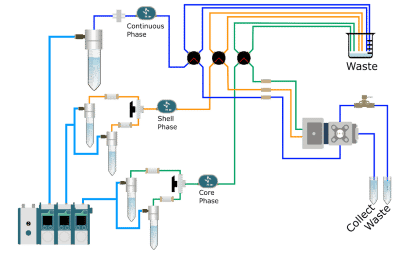
Methods for production of monodispersed double emulsions
- First, the phases are filtered (pore size 0.2 µm). Then, a simple emulsion of the shell phase is produced by closing the core phase and adjusting the flow rates of the continuous phase and the shell phase.
- Once the single emulsion is produced, the double emulsion process is performed by turning the core phase valve in the reservoir position towards the RayDrop and adjusting the flow rates.
- Once an optimal and stable flow rate is reached, the production of the double emulsion is started.
- After a few seconds, we can collect our droplets in their corresponding Falcon tubes for further analysis.
Visit our protocol for encapsulation of cells, where we detail, step by step, how to perfom double emulsion generation using the cell encapsulation Platform.
Results: Droplet Generation
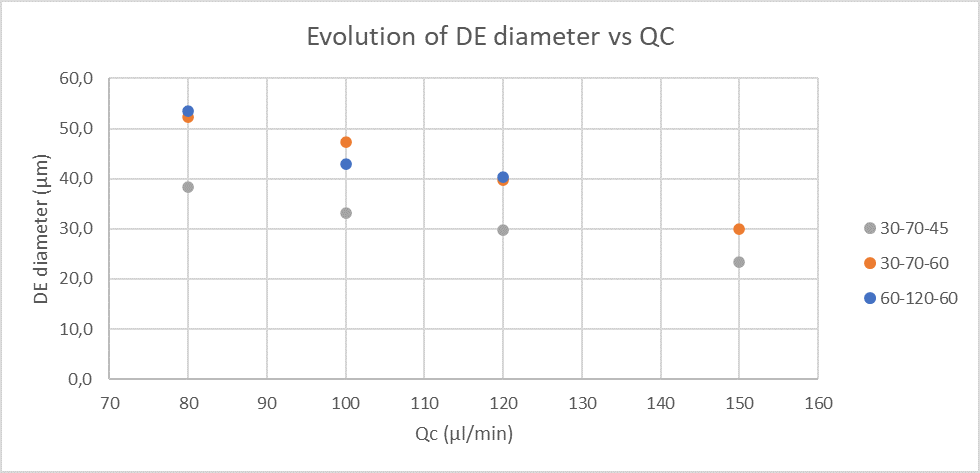
The size of the double emulsion produced depends on the Raydrop configuration and the flowrates of the different phases. However, for a specific Raydrop configuration, the continuous phase flowrate has the most impact on the double emulsion size.
Figure 1 presents the evolution of double emulsion outer diameter produced for the three Raydrop configurations with respect to the continuous phase flowrate.
Table 1 (in the application note, page 8) summarizes the flow rates of each phase, the outer diameters of the double emulsion and the respective coefficient of variation
Cellular encapsulation technique with Small Double Emulsions
Materials
While the shell phase is composed of dSurf (HFE7500 + 2% biocompatible surfactant), the core phase consists of water with 0.5% Fluorescein.
Finally, the continuous phase is composed of water with 2% Tween20.
Methods
- First, the shell and core phases (pore size 0.2 µm) and the cell solution (cell strainer pore size 40 µm) are filtered and the corresponding reservoir is filled.
- Then, a simple emulsion of the shell phase is produced by adjusting the flow rates of the continuous phase and the shell phase.
- Once the single emulsion is produced, the cell encapsulation process is performed by turning the core phase valve in the reservoir position towards the RayDrop and adjusting the flow rate.
- After a few seconds, we can collect our encapsulated cells in their corresponding Falcon tubes for further analysis.
Results: efficient encapsulation of cells
Once the encapsulation process is completed, the generated double emulsions are visualized under a microscope to check their monodispersity and stability.
We used fluorescent labeling to demonstrate the chemical functionality and uniform incorporation of human PBMC cells into these double emulsions.
For this purpose, we used the Cy5 dye, the TRITC dye and the fluorescein fluorophore (FITC) to stain the entire medium in which the cells were found.
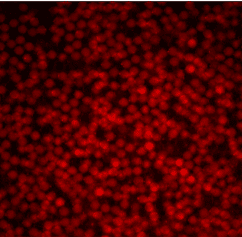
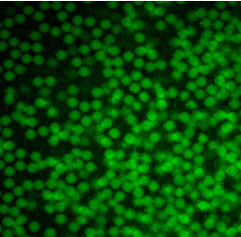
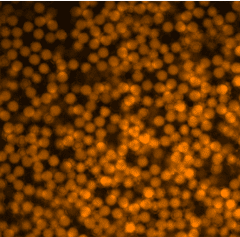
Figure 2. Staining of droplets and their respective media with different fluorophores.
On the other hand, CellTrace™ Violet Cell is used to both visualize the successful incorporation of cells into the droplets, and to verify the functionality of these droplets. CellTrace™ Violet Cell is used for in vitro and in vivo labeling of cells to trace multiple generations using dye dilution by flow cytometry.
As seen in the figure 3, the droplets have uniform sizes and fluorescence intensity. The fluorescence among droplets within each fluorophore appears uniform for each condition.
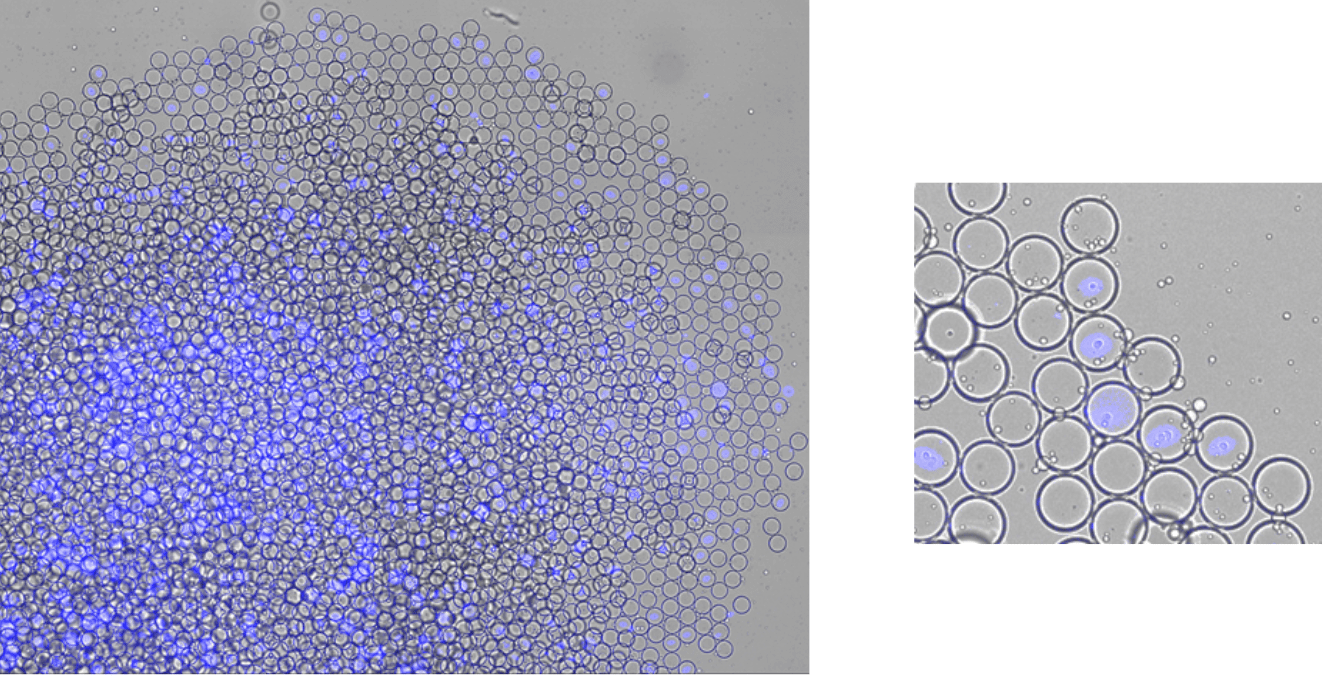
Courtesy of Functional Immune Repertoire Analysis at ETH Zürich.
Conclusion
In this application note, we have demonstrated that the Raydrop is able to produce monodisperse double emulsion with an outer diameter below 60 µm. We also demonstrate that the Cell Encapsulation Platform can encapsulate cells in w/o/w double emulsion with a precise control of droplet size.
Human PBMCs were encapsulated in water-oil-water monodisperse double emulsion of 52 µm using a 60-120-60 Raydrop configuration. Other RayDrop configurations, with different nozzle dimensions, are available to target different ranges of droplet sizes, and therefore different ranges of cell sizes. This is due to the versatility and flexibility of the RayDrop, which enables us to easily change configuration and change capillary size.
References
- Brower, K.K. et al. (2020) “Double emulsion picoreactors for high-throughput single-cell encapsulation and phenotyping via FACS,” Analytical Chemistry, 92(19), pp. 13262–13270. Available at: https://doi.org/10.1021/acs.analchem.0c02499.
- Wang, W., Zhang, M.-J. and Chu, L.-Y. (2014) “Microfluidic approach for encapsulation via double emulsions,” Current Opinion in Pharmacology, 18, pp. 35–41. Available at: https://doi. org/10.1016/j.coph.2014.08.003.
- Yan, J. et al. (2013) “Monodisperse water-in-oil-in-water (w/o/w) double emulsion droplets as uniform compartments for high-throughput analysis via flow cytometry,” Micromachines, 4(4), pp. 402–413. Available at: https://doi.org/10.3390/mi4040402.
- Lim, S.W. and Abate, A.R. (2013) “Ultrahigh-throughput sorting of microfluidic drops with flow cytometry,” Lab on a Chip, 13(23), p. 4563. Available at: https://doi.org/10.1039/c3lc50736j.
- Brower, K.K. et al. (2020) “Double emulsion flow cytometry with high-throughput single droplet isolation and nucleic acid recovery,” Lab on a Chip, 20(12), pp. 2062–2074. Available at: https://doi.org/10.1039/d0lc00261e
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Microfluidics Article Reviews A mRNA encapsulation platform integrating Fluigent’s FlowEZ Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes What is the best method for Microencapsulation of Bacteria and Yeast in Small Double Emulsions? Read more
-
Microfluidic Application Notes Encapsulation of multiple emulsions in a single droplet Read more
-
Microfluidics White Papers Droplet-based Microfluidics Read more
-
Microfluidics case studies The Hebrew University: Encapsulation and culture in 3D hydrogels for Single cell sequencing Read more
-
Microfluidic Application Notes E. Coli Culture in Droplets Using dSURF Fluorosurfactant Read more