Cartilage-on-a-chip, an example of complex mechanical stimulation using Fluigent’s technology
In understanding the processes responsible for osteoarthritis or other cartilage diseases, exploring the response of chondrocytes to external mechanical and chemical stimulations can lead to innovative therapies. While traditional approaches have allowed the scientific community to highlight some particularly interesting behaviors of chondrocytes, their lack of physiological relevance can lead to false conclusions.
In this application note, we report a new method for 3D chondrocyte culture using a microfluidic platform associated with Fluigent products, enabling researchers to create a relevant cartilage model subject to complex mechanical stimulation.
This application note was made possible thanks to Séverine Le Gac and her team at Applied Microfluidics for BioEngineering Research (AMBER). For more information, visit the team website.
What’s the value of simulating complex mechanical stimulation?
Articular cartilage is a type of connective tissue located at the end of long bones to protect them from friction and to help them support heavy loads (Fig 1). It is usually bathed in synovial fluid, and is therefore subject to mechanical and biochemical activity.
Biochemical or mechanical breakdown of the cartilage results in a disease called osteoarthritis, the most common joint disease, which affects millions of people worldwide. Numerous models have been developed by scientists to understand the processes underlying this disorder, with the goal of finding an optimized cure. However, current methods usually rely on 2D cultures of chondrocyte cells in static conditions, which is physiologically far from the in-vivo 3D assembly of the cells inside an extracellular matrix constantly exposed to fluid flows.
Microfluidics is of growing interest in the scientific community, as it allows for perfused 3D culture of cells, enabling the development of highly physiologically relevant models in systems referred to as organ-on-a-chip. In these models, complex mechanical stimulations can be reproduced by the controlled injection of fluids into the system.
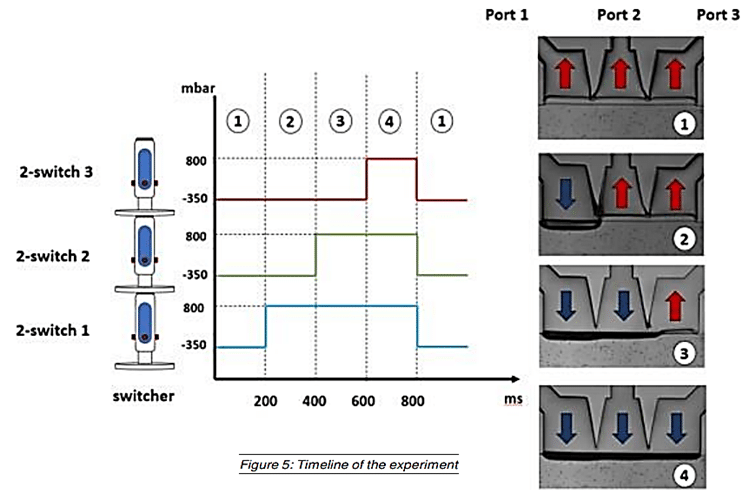
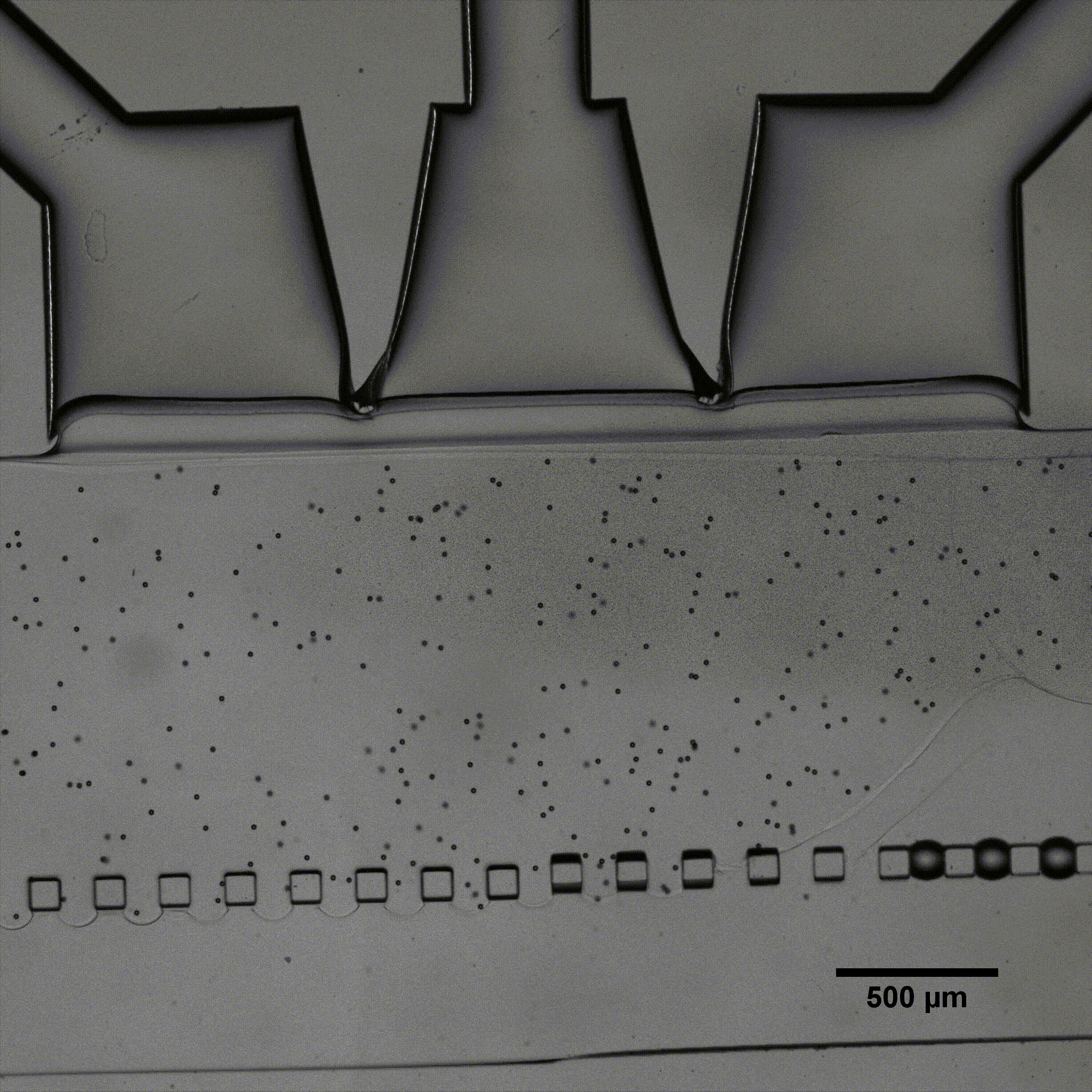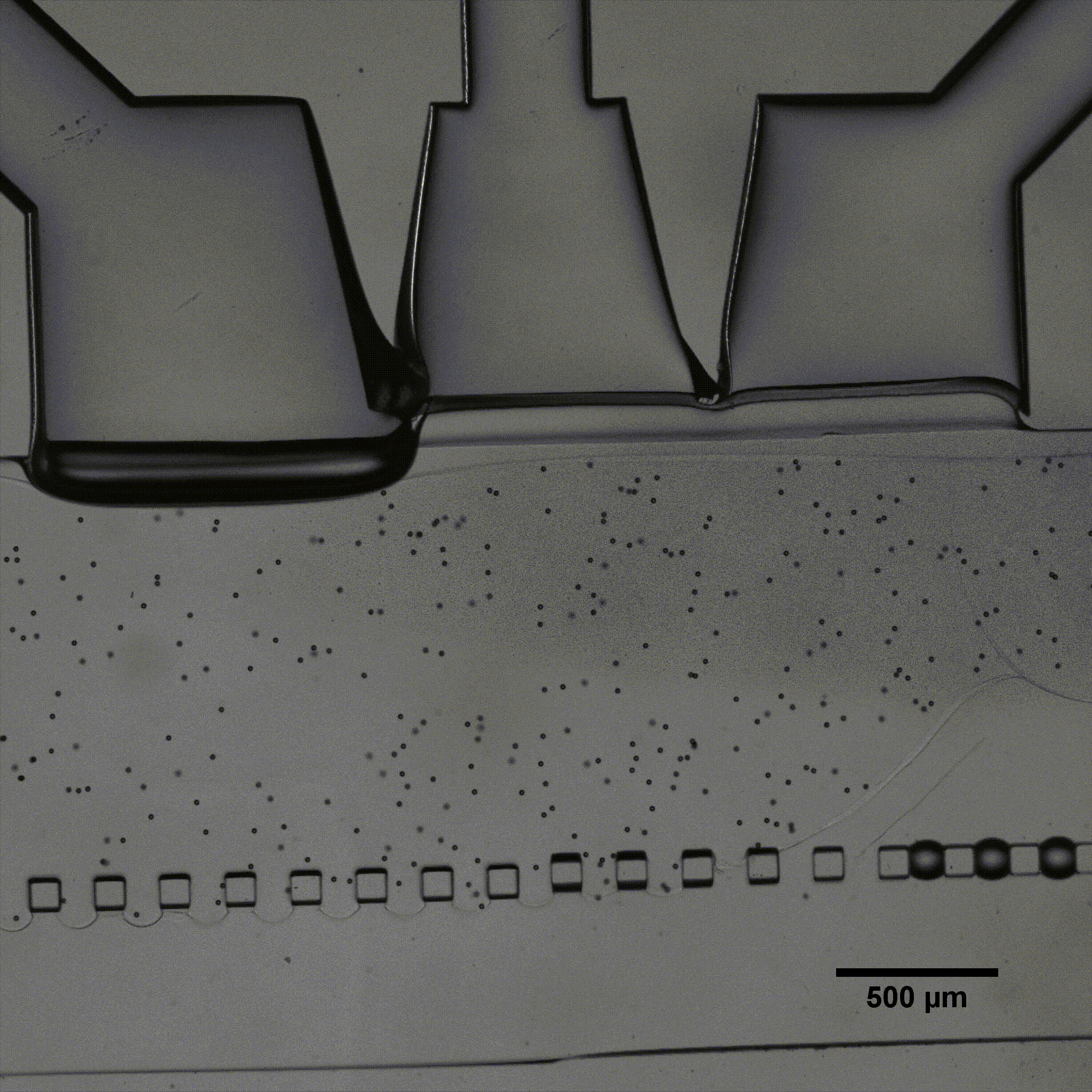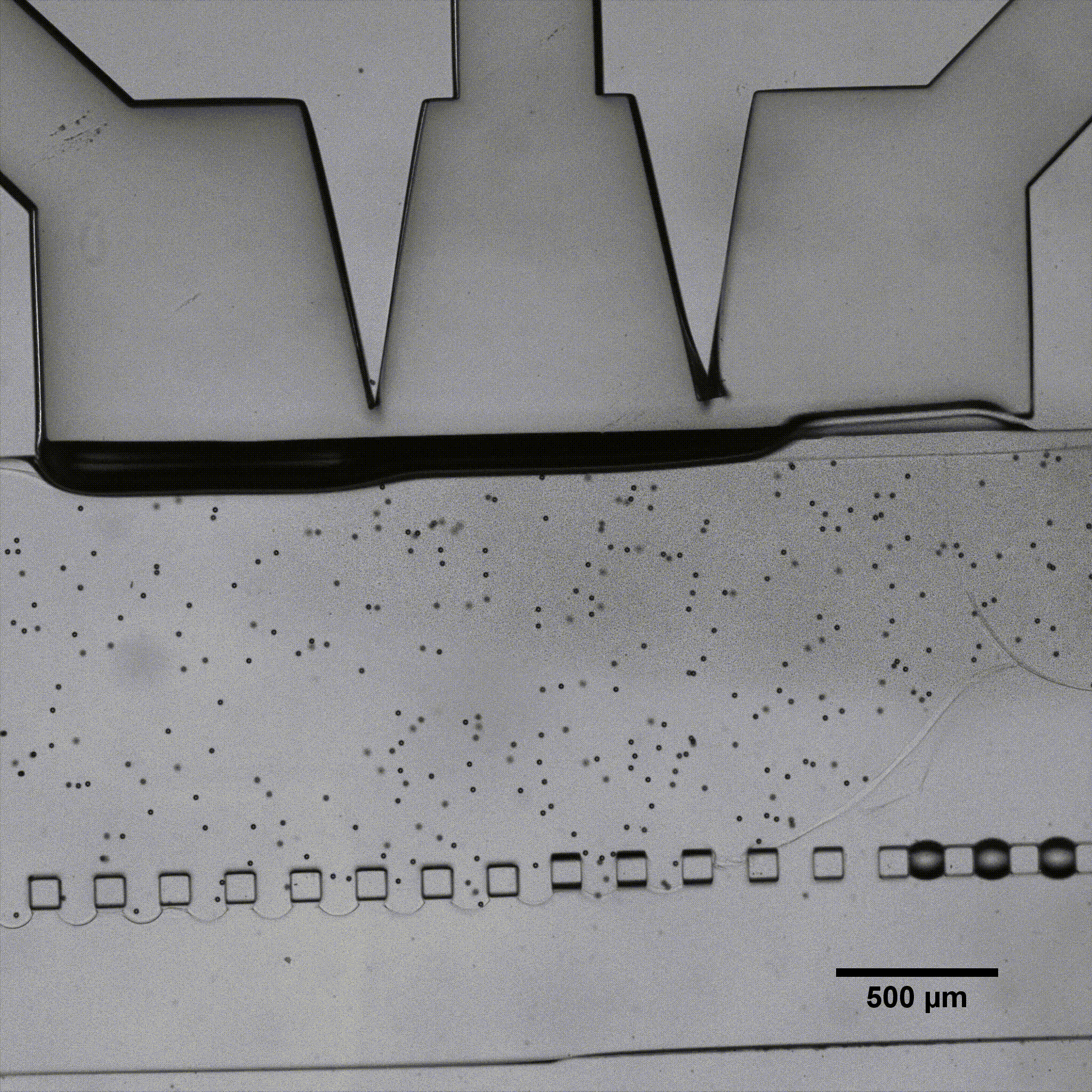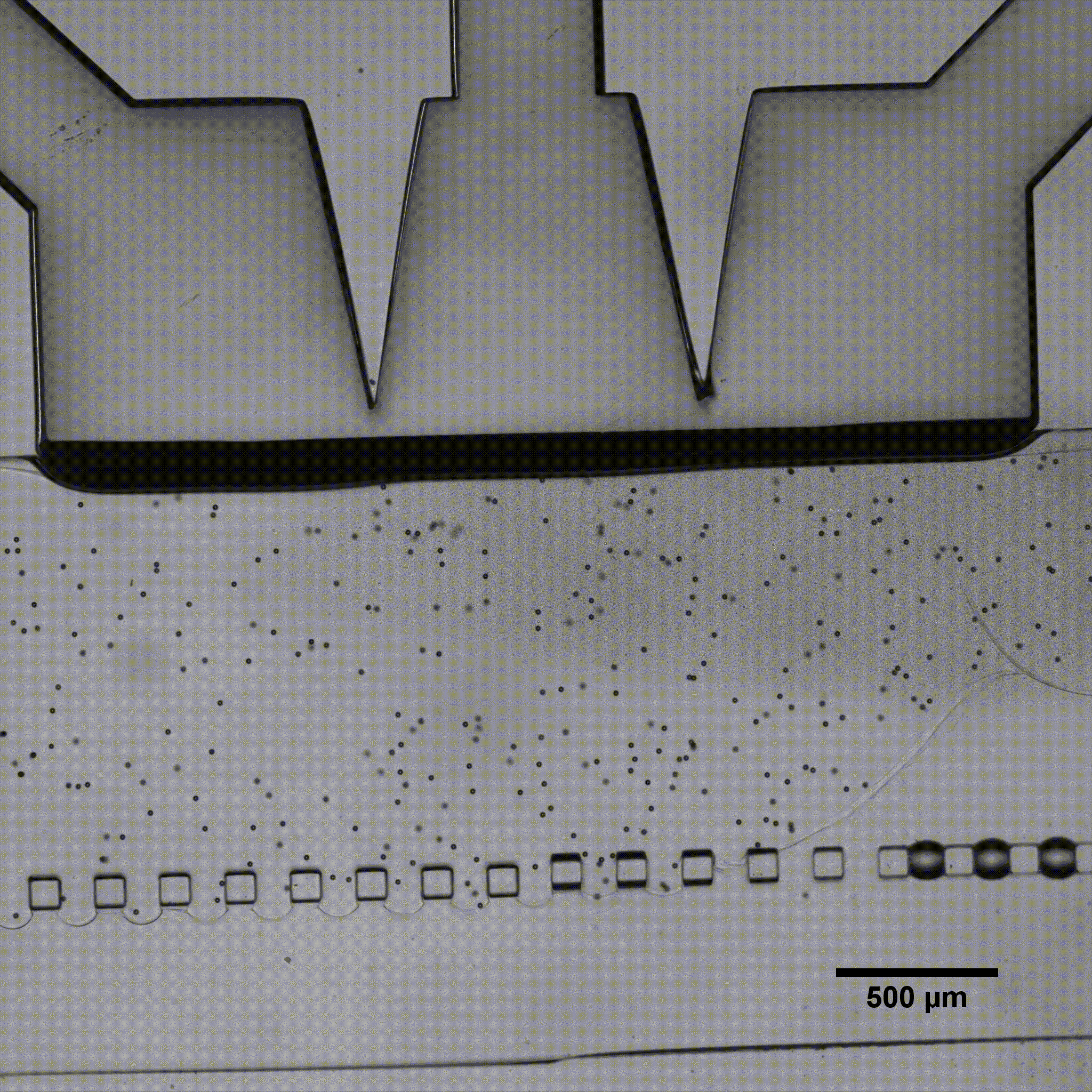
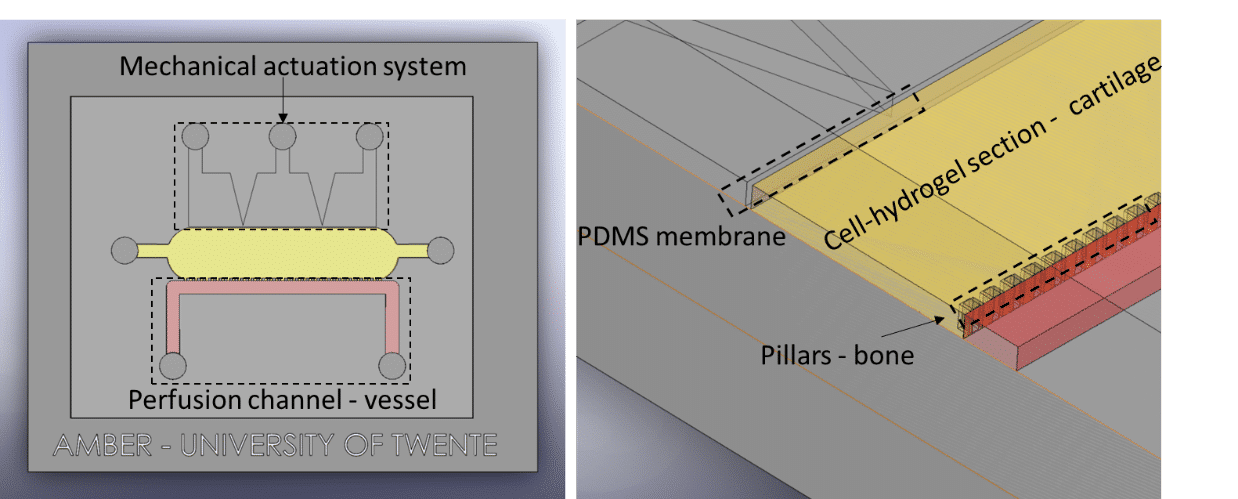
Séverine Le Gac’s team developed a cartilage model in which an agarose hydrogel containing cultured human chondrocytes is subject to compression through the pressurization of actuation chambers to trigger membrane deformation (Fig 2). The effect of this advanced mechanical stimulation on cells was observed.
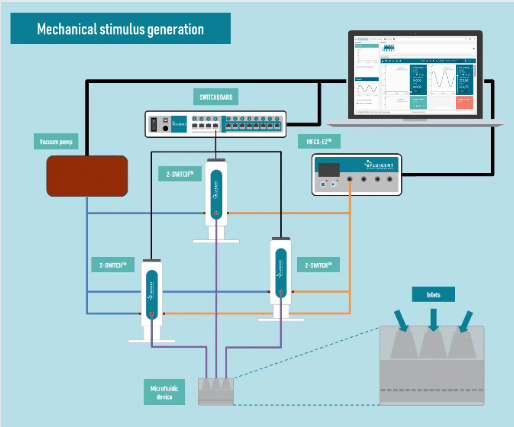
Generating complex mechanical stimulation
Fluigent instruments
Pressure generator: An MFCS-EZ pressure controller was used for fluid injection with an excellent response time and high stability. Pressure ranging from 0 to 1500 mbar was generated.
Valves: Three 2-switch valves were connected to the pressure generator and a vacuum pump to apply positive or negative pressure to the three actuation chambers and generate complex mechanical stimulation. The valves were connected to a switchboard to allow monitoring on the computer.
Software: Our automation software was used to predefine and automate the pressure levels, enabling the production of complex patterns inside the chip.
The Fluigent setup is shown in Fig 3.
Other instruments
Microfluidic chip: Conventional soft-lithography techniques were used to fabricate the chip, made of poly-dimethyl-siloxane (PDMS) and bonded to a PDMS-coated glass slide. This device allows for 3D culture of chondrocytes under dynamic conditions (nutrient supply) and enables the application of a complex mechanical stimulation by deforming the PDMS membrane using pressure (Fig 4).
Camera: An ORCA-flash 4.0 LT from Hamamatsu Photonics was used to visualize the effect of pressure on the PDMS membrane.
Methods to create complex mechanical stimulation
Experiments were done to verify the cellular viability of chondrocytes upon application of advanced mechanical stimulation. First, a culture of chondrocyte cells isolated from a patient undergoing knee replacement was done. Then cells were cultured inside the microfluidic chamber, with or without (control) mechanical stimuli via application of pressure in the actuation chambers. Several pressures were tested. Finally, a live/dead assay was performed using fluorescent probes to assess cell viability.
Partial results
It was demonstrated that cell viability was not affected by the membrane deformation. The ratio of living cells to dead cells was similar in the case of chondrocytes subject to complex mechanical stimulation and in the control test.
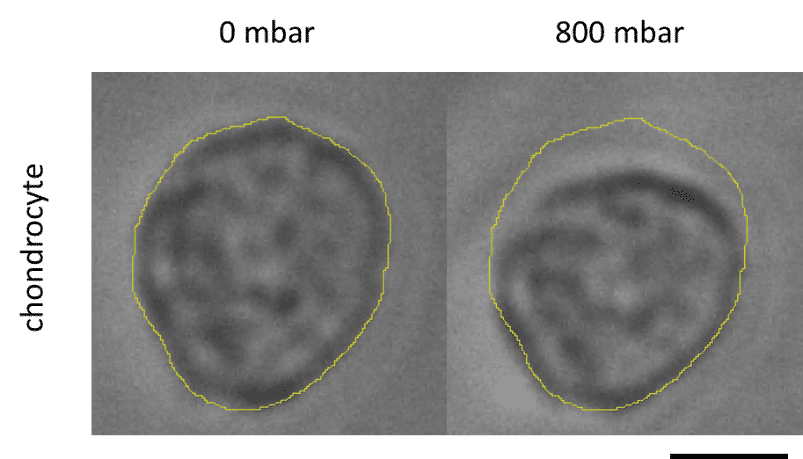
In addition, a study was performed of the cell deformation due to application of pressure in the actuation chambers, and showed that it was not uniform in the matrix, with a maximum deformation value of 13% close to the membrane, decreasing along the matrix’s depth (Fig 6).
Conclusion
Fluigent products were used as an essential tool to develop a physiologically relevant 3D cartilage model and create a complex mechanical stimulation. The application of pressure in the actuation chambers was demonstrated to have an effect on cell shape, but no effect on cell viability was found. More analysis will be done to evaluate the response of chondrocytes to this deformation.