Automating calcium imaging in neural cells with Fluigent’s Aria
Following our application note on neuron cell immunolabeling, we’ve produced a new application outlining how the Aria, in combination with the microfluidic bidirectional valve, automates cell stimulation for neuron calcium imaging. This process allows users to localize a field of interest under the microscope for data acquisition, while maintaining minimal cell damage and reducing both time and effort.
Calcium imaging: Understanding neuronal communication activity
Assessing neuronal activities in vitro is crucial for understanding the functionality, health, intrinsic properties, and responses to various stimuli of neuronal cultures. Calciumplays a significant role in cell signalingas an indicator for tracking neuronal electrical activities. This method is capable of detecting single action potentials in individual neurons. By utilizing fluorescent markers that react to the binding of Ca+ ions, one can optically measure shifts in calcium concentration within neurons and neuronal tissue.
Imaging calcium offers insights into neuronal events, network synchrony, response amplitudes, and event frequencies, allowing for the comprehensive characterization of culture functionality.
Visualizing Neuronal Activity
By utilizing super resolution fluorescence microscopy with calcium imaging one can indirectly measure neuronal activity. This is achieved by monitoring calcium influx during action potentials. The automation of the process offers control over the cellular environment while capturing neuronal activity.
Automated Imaging with Fluigent’s Aria Platform
Below is an automated calcium imaging protocol using Fluigent’s Aria platform. The Aria is an automated perfusion device, able to deliver up to 10 solutions into 1 or 9 microfluidic chips or chambers this configuration maintains the stability of neuronal cultures for prolonged recordings without the need to move the sample and the field of interest. This application note showcases the potential of the Aria in streamlining experimental procedures, enhancing experimental throughput, and ensuring the reproducibility of results. Through real-time monitoring of calcium dynamics, researchers gain valuable insights into neuronal circuit dynamics, neurodegenerative diseases, and drug effects, advancing our understanding of neuronal function.
This document is written by Maxime Poinsot, a PhD student at Institut de Neurosciences de la Timone & Fluigent. The application employs standard reagents, making it accessible and practical for researchers in the field.
Previous Application Note on the topic:
Automated Calcium Imaging Protocol
Materials
- Cells & neuron stimulation reagents:
- Progenitor neuron cells from rat embryos at 17.5 days of gestation
- AAV5.SYN.GCaMP6f for transduction of the striatum
- Tetrodotoxin (TTX) 5μM in water for cellular cultures
- Microfluidic chip: The chip (Figure 1) used for this application note is fabricated using PDMS (Polydimethylsiloxane) with a curing agent ratio of 1 part curing agent to 10 parts of the base silicone, utilizing Sylgard 184 as the base material.
- Live Imaging Super Resolution Microscopy TIRF: ZEISS Elyra 7: The confocal microscope employed provides high-resolution imaging for neuron cell visualization. Recordings were made using a 20x oil-immersion objective and a 488 nm laser at 70% intensity with a 200 ms exposure time every 200ms for 1 minute.
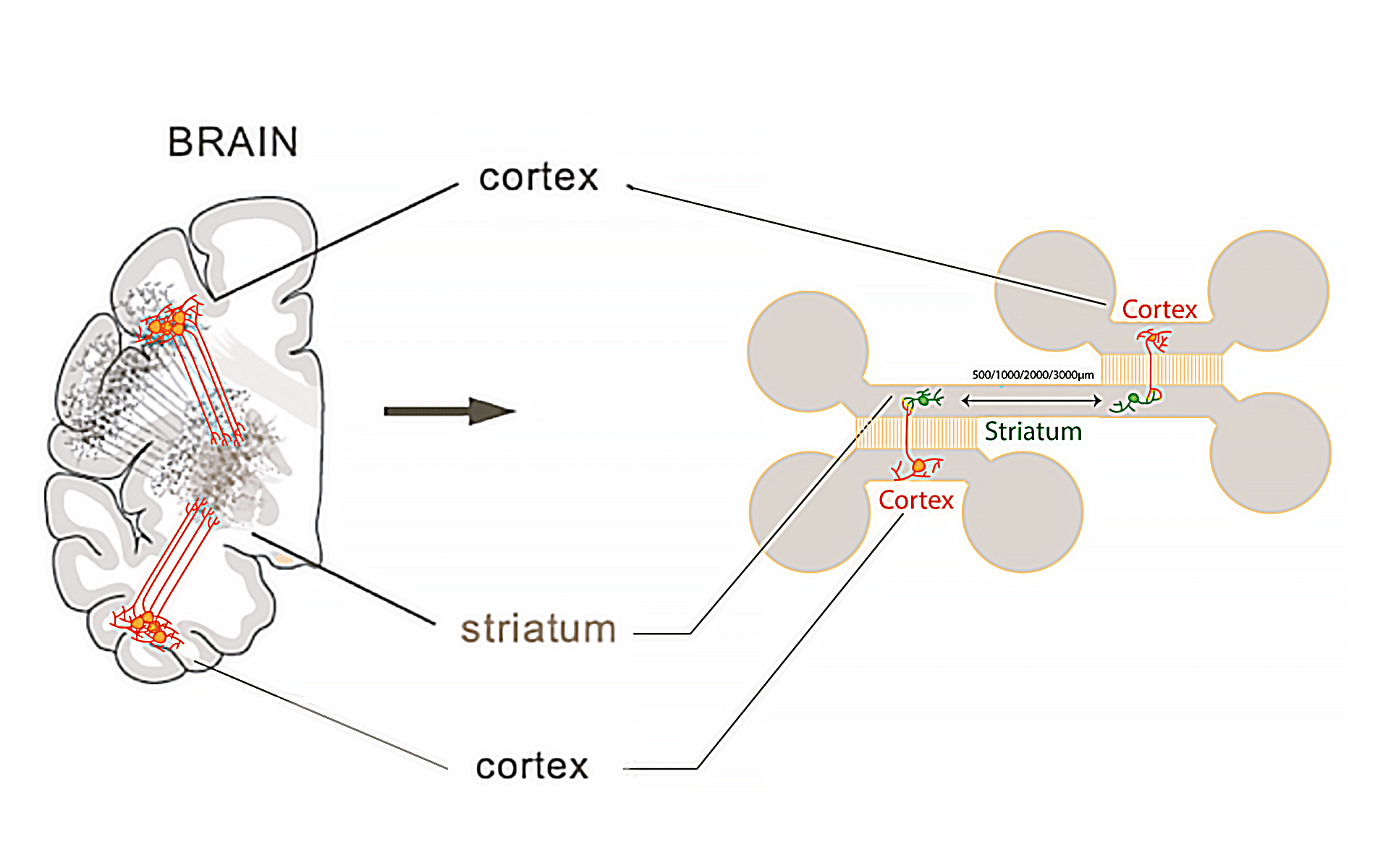
Protocol: Imaging Neurons Using the Aria
- After culturing neuron cells for 21 days inside the chip or chamber, the device is placed under the microscope and the preferred field of interest is localized.
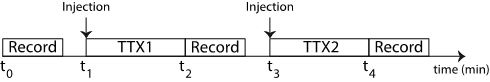
- The basal calcium activity of the striatum is measured every 200 ms over 1 minute T=0.
- Using Aria, the direct cortex culture medium is replaced by the medium containing TTX, followed by an incubation time of 5 minutes (t1).
- The calcium activity of the striatum is recorded every 200 ms for 1 min (t2).
- Again, the culture medium is replaced by the medium containing TTX, followed by an incubation time of 5 minutes (t3).
- The calcium activity of the striatum is recorded every 200 ms for 1 min (t4).
The protocol is summarized below (Figure 2).
Results
Figure 3 displays progenitor neuron cells within the chip following various stimulations. The images depict recorded striatal neurons alongside their normalized amplitude traces. Administration of TTX into the direct Cortex 1 reveals persistent activity in the striatum, indicating functional connectivity from the indirect cortex 2 to the recorded striatum. Conversely, administering TTX into both direct Cortex 1 and 2 results in no recorded neuronal activity.
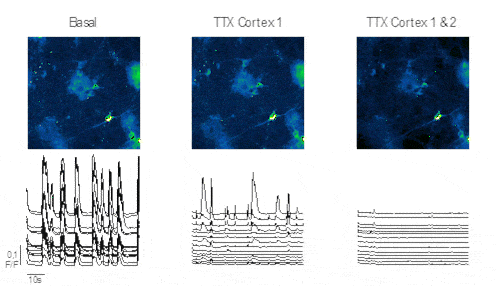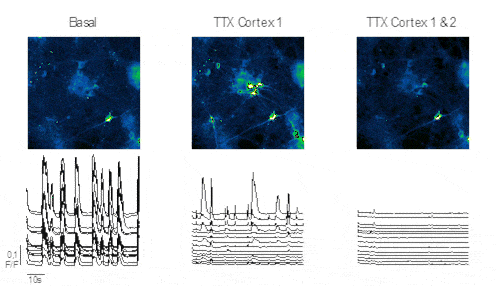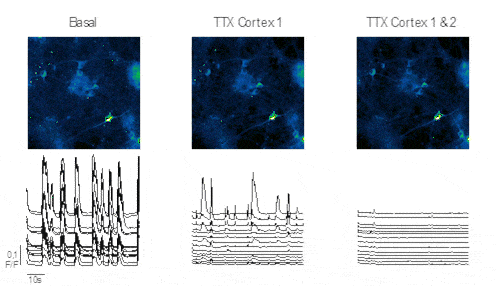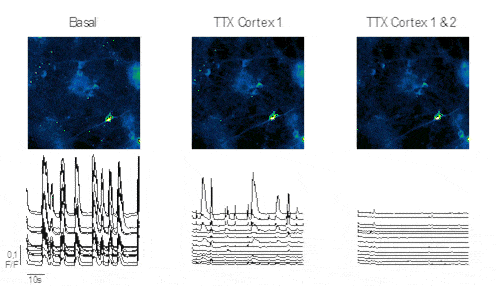
Conclusion
Using the Aria we fully automated a calcium imaging in neurons protocol, expediting numerous microfluidic experiments. This ensured stable recordings, enhancing reliability and reproducibility. This streamlined approach advances our understanding of neuronal circuit dynamics and offers a transformative solution for microfluidic calcium imaging challenges.
Related product
Related content
-
Microfluidics Case Studies Multi-parametric Functional Assays of Cardiac Organ-on-a-Chip using Live-cell Microscopy and Fluigent, Aria Read more
-
Expert Reviews: Basics of Microfluidics Optimizing Microfluidic Perfusion: Best Practices and Innovations Read more
-
Microfluidic Application Notes Automating Neuronal Cell Immunofluorescence in Microfluidic Chips Read more
-
Microfluidics Case Studies Automated immunolabeling to perform highly multiplexed tissue imaging with ARIA Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Expert Reviews: Basics of Microfluidics How to choose a microfluidic chip Read more