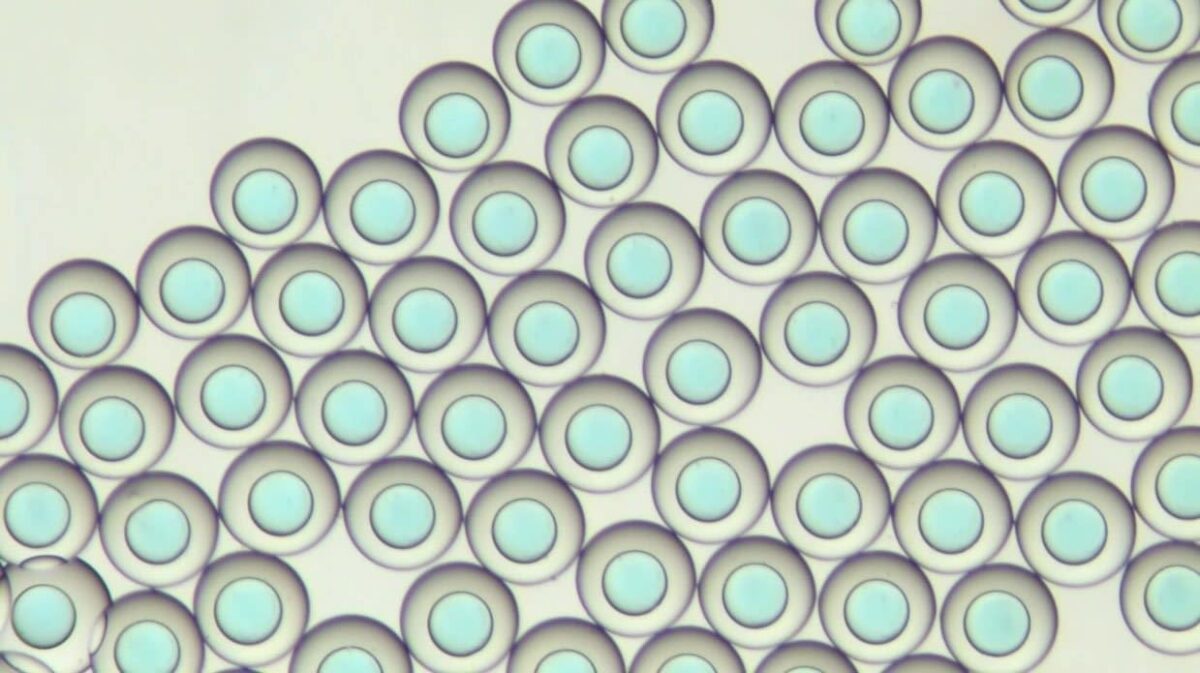
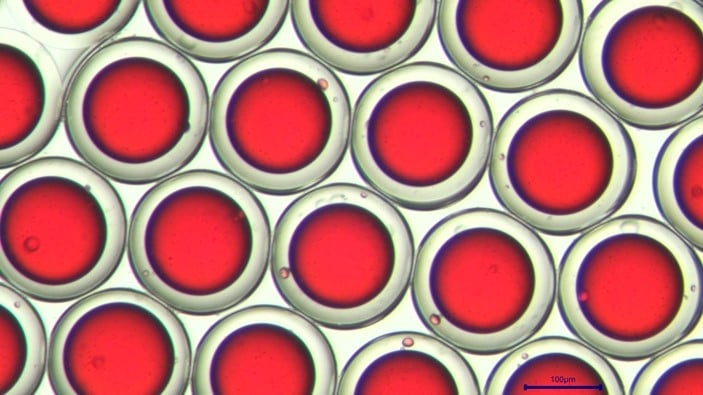
Down to 2% monodispersity
Encapsulation Platform for FACS
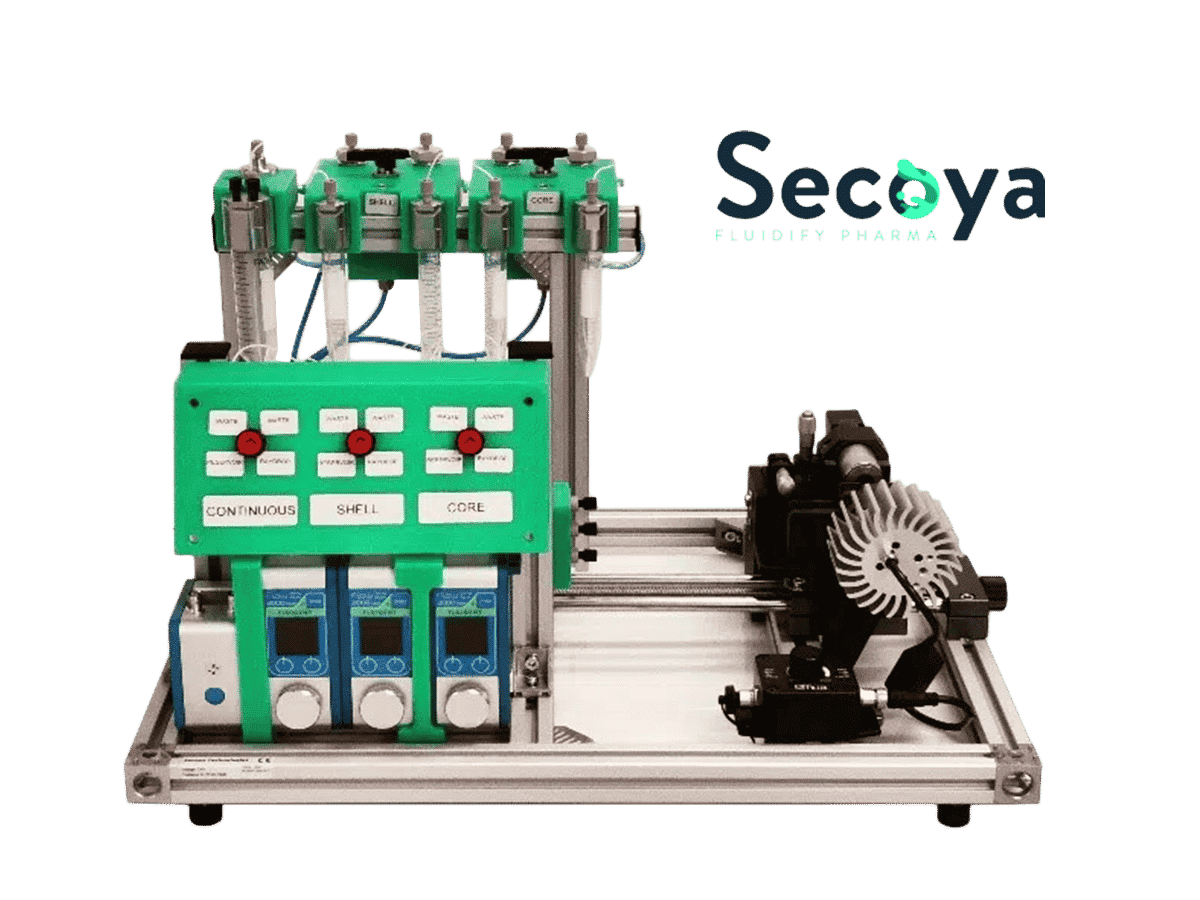
An encapsulation platform for FACS experiments
The cell encapsulation platform for FACS (Fluorescence-Activated Cell Sorting), developed and manufactured by Secoya using Fluigent’s flow control equipment and Secoya's Emulsion Technology, is a complete system for the high throughput encapsulation of complex and individual cells within highly monodisperse double emulsion droplets small enough (<90 µm) for further analysis.
This fast and easy system allows for the encapsulation of cells in aqueous core (such as medium, PBS) and oil shell (such as HFE 7500) double emulsions, acting as a powerful tool for biochemical and cellular assays., The platform enables the isolation of each cell within microreactors, highlighting its characteristics and concentrating the signals to measurable levels to obtain meaningful biological data.
Moreover, the platform is an ideal tool for FACS experiments. This technique produces the sorting of cells in a heterogeneous population via the detection of specific fluorescent signals. It is widely used today but has limitations. A binding protocol must be done in a quasi-systematic way to detect the cells that dissolve in the media and the secretion of the cells influence each other. Using the encapsulation platform for droplet sorting enables users to overcome these issues since it creates the individualization of cells for efficient single-cell studies while considerably reducing sample requirements. It is thus possible to study rare cells whose availability is limited such as primary cells obtained from patients. In other words, using the platform for FACS minimizes the need for large cell cultures and expensive reagents.
To learn more about double emulsion generation for FACS sorting, read the application note.

- High precision
- Flexible
Adjustable droplet size and shell thickness
- Robust
Continuous production
- Easy to use
Intuitive interface and ready-to-use system
Features of the cell encapsulation platform for flow cytometry
➤ Fluigent Precision and Flexibility
Produce robust and highly monodisperse emulsions using Fluigent’s pressure-based flow controllers and the Raydrop while accurately controlling the size of your droplets and shell thickness.
➤ Start emulsions production right away
The system is a fully equipped, mounted and controlled tool to create double emulsions with little setup time.
➤ Complete and easy-to-use engineered system
Our organized system requires easy priming and cleaning processes for better performance. It includes dedicated optics for optimized droplet visualization at a high frequency.
➤ Innovative and much sought-after application for cell analysis
The system is an easy-to-use platform for creating single-cell encapsulations into double emulsion droplets compatible with high-throughput screening and FACS experiments.
The encapsulation platform for FACS allows:
- Elimination of the risk of cross-contamination.
- Fast and efficient mixing of the reagents that occurs inside droplets.
- Ability to work with cells of limited availability.
Description
The cell encapsulation platform includes an organized flow path with pressure controllers, filters, flowmeters, and valves to facilitate the start-up, shut-down, and cleaning of the system between runs. Dedicated optics are included to optimize the visualization of droplet production at high generation frequency.
What is FACS technology?
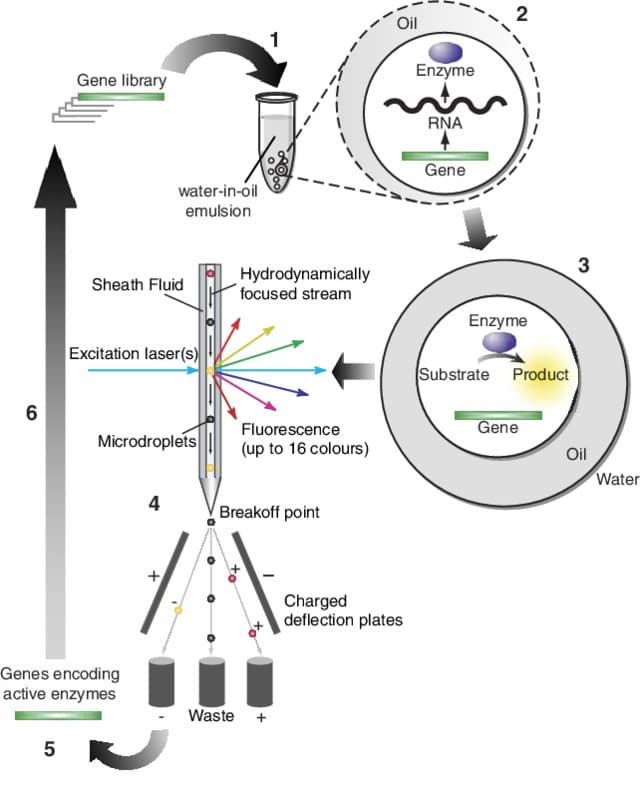
In the field of biology, FACS stands for Fluorescence-Activated Cell Sorting. It is a technology aimed at separating and isolating certain cells from a heterogeneous population based on their fluorescent properties. This fluorescent signal allows users to translate the production of certain molecules of interest or the expression of specific phenotypes. The process of this technique is to label the cells with fluorescent markers such as antibodies or dyes which have the role of binding to certain structures or molecules of the cell in a specific way. These labeled cells can then be passed through the flow cytometer, an instrument allowing for the detection and measurement of the fluorescence emitted by the cells. This fluorescence detection will allow the flow cytometer to sort the cells into different populations, allowing the researcher to collect and analyze these different groups of cells separately.
Using FACS enables the scientist to perform an efficient sorting with a limited number of doublets or multiple cells being sorted together and from there, the characterization of cellular heterogenicity and the rare cell subpopulation.
Through our microfluidic technology, the encapsulation platform for FACS allows for the generation of samples, which can be processed in flow cytometry.
How to use the platform for FACS experiments?
Microfluidics can be associated with FACS technology to improve results. Working with microliter volumes allows for a considerable reduction in cost since the volume of samples required is greatly reduced. For instance, it minimizes the need for large cell cultures.
In addition, the use of encapsulation methods in droplets allows users to confine the fluorescence signal, avoiding the use of chemicals, and to perform bioassays at the single cell scale. Single cell or bacteria growth in drops can also be considered.
As the encapsulation platform for droplet sorting produces double emulsions to encapsulate cells (from 5 µm to 40 µm), the limitations of the FACS are overcome. The first limitation is that the secreted molecules are generally detectable only if a binding protocol has been performed. In the opposite case, they remain dissolved in the surrounding medium. The second is that the secreted cells significantly impact each other. Combining droplet microfluidics and FACS technology then appears to be a major advance for research and allows users to obtain more reliable and reproducible data.
Our selection of webinars on double emulsion for FACS
Encapsulation of fluorescent bacteria in double emulsions for FACS
Learn how to use our double emulsion platform to encapsulate bacteria in W/O/W DE and discover the DE sorting efficiency using FACS.
Webinar summary:
- Introduction to FACS sorting: technology, advantages, and drawbacks
- Presentation of the platform: encapsulation of single bacteria in DE
- Microscopy characterization and FACS sorting
- Interactive Q&A session
Single cell encapsulations compatible with FACS sorting, API encapsulations in biocompatible polymers, and more.
Until now, performing high efficiency encapsulation processes continuously has been challenging, as existing technologies have limitations (low reproducibility, the need for multiple surface coatings, poor size distribution, etc). The Raydrop encapsulation platform from Secoya solves these issues by providing an easy-to-use system that produces highly monodisperse W/O/W or O/W/O single or double emulsion from 15 µm to 400 µm.
Webinar Summary:
- Theoretical presentation of the Raydrop’s encapsulation process
- Case study: encapsulation of cells, yeast, and bacteria in a small double emulsion for FACS sorting
- Case study encapsulation of API in microbeads and microcapsules
- Q&A
Cell encapsulation platform: Examples of use
Standard cell encapsulation platform
The standard cell encapsulation platform allows for the encapsulation of single cells for high throughput screening applications involving drug discovery, toxicity testing, various ‘omics’ studies and rare cell analysis1,2.
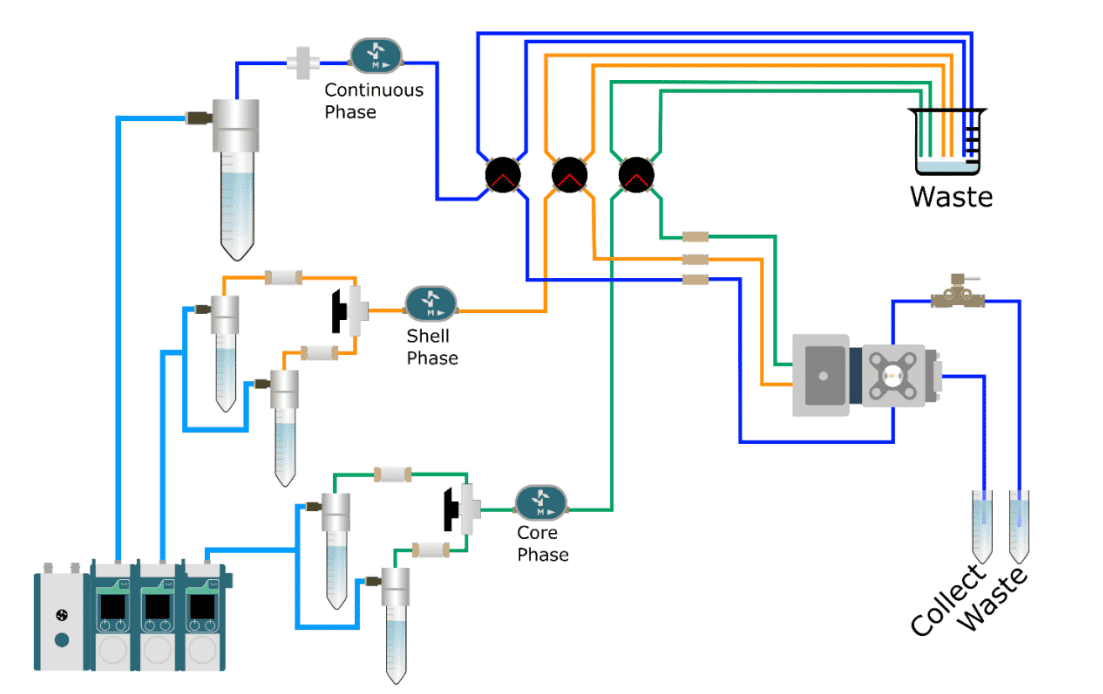
How to inject a small sample?
The encapsulation platform for flow cytometry with an injection loop is a tool capable of greatly improving working conditions as it handles fluid volumes, as low as a few dozen microliters. Adding the L-Switch is advantageous in many scientific and medical applications, making it possible to work with rarer cells.
For instance, researchers working on stem cells are often challenged by their low availability. Indeed, because of their limited distribution in time (available only during a period of human life, difficulties to self-renew into stem cells after differentiation) and space (often nested in environments favorable to their functioning, protection and regulation, which limit their dispersion in the organism) and their delicate and expensive conditions of culture, their large-scale production is not possible. Thus, to be able to work with small volumes of culture and thus a low number of cells is advantageous.
Another example is the use of primary cells, directly collected from the patient and useful for personalized medicine. Acquiring this type of cells can be logistically challenging and the available volume can also be limited. The cell encapsulation platform’s injection loop solves this predicament by enabling researchers to work with small volumes of patient-derived cells.
Finally, using this platform for FACS also allows users to limit the use of expensive reagents and to limit wastes, unlocking new possibilities in the field of biomedical research and personalized medicine.
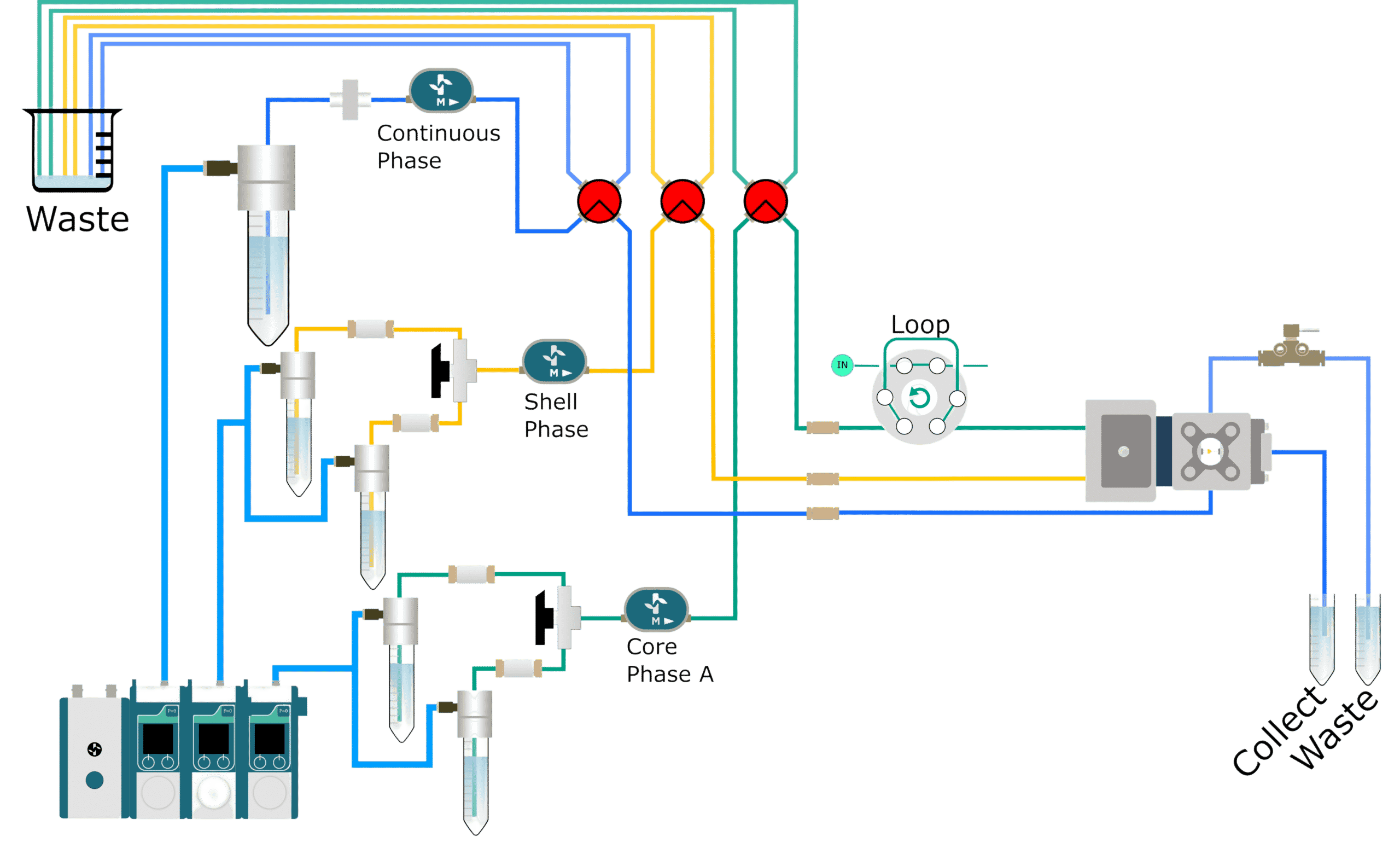
How to add a reagent?
In many microfluidic procedures, an integrated tooling for mixing is essential for appropriate functionality in a broad range of applications.
The usual encapsulation platform for drop allowing users to integrate a new phase for optimal agent mixing. It is recommended to mix two solutions in a fluidic path before encapsulation. After the precise formation of self‐ assembled lipid or polymeric particles by microfluidic mixing, the encapsulation of active molecules is loaded into synthesized particles.
The efficiency of many biosensors and the possibility of investigating reaction kinetics with quick time resolution depends on controlled mixing. It can be also used to encapsulate cells with barcoded beads for Next generation sequencing (DropSeq), for enzymatic tests, or phenotyping5.
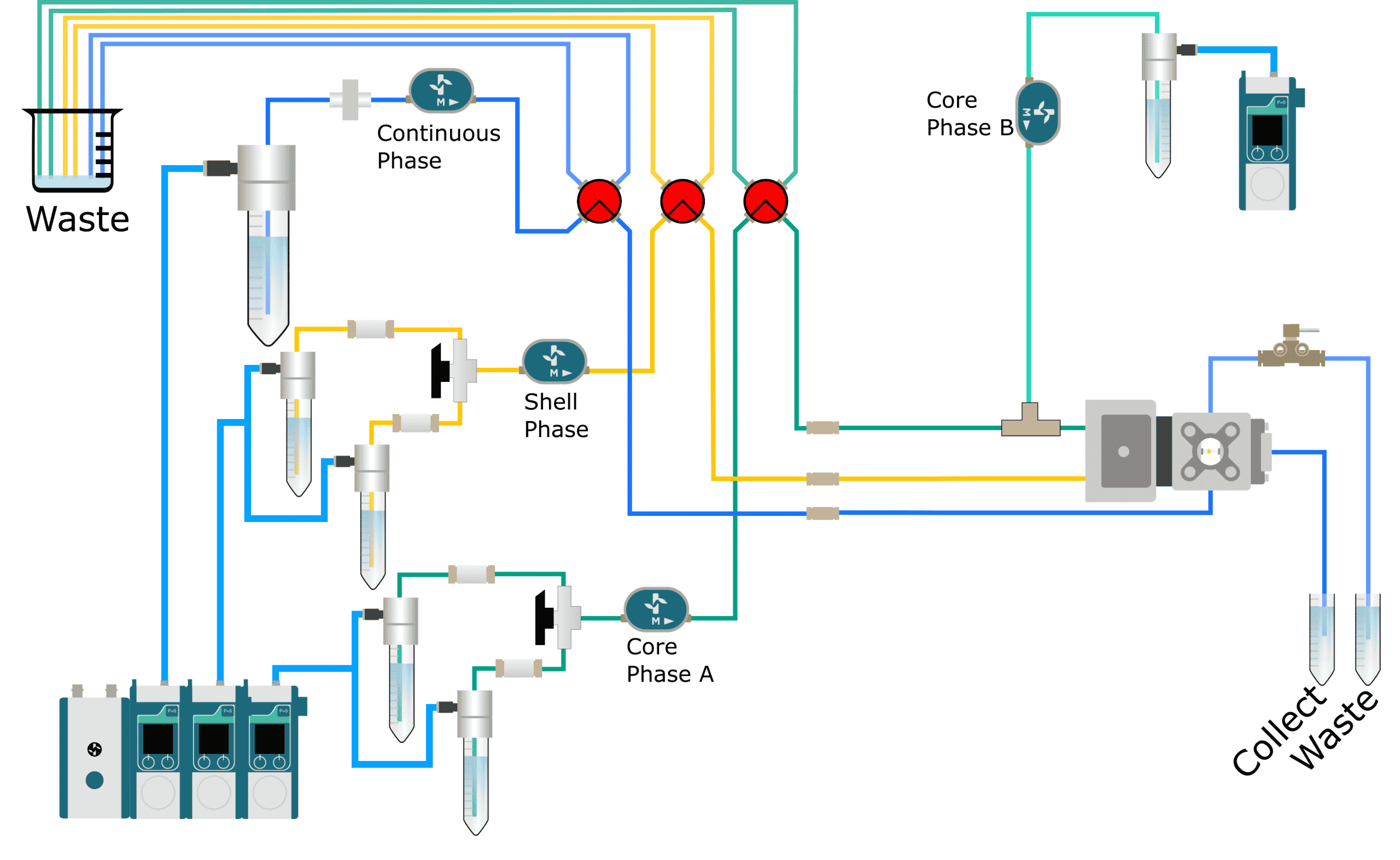
Which applications can use the encapsulation platform for FACS?
High-throughput screening: Cell sorting
Cell encapsulation can improve high-throughput screening outcomes since the highly monodisperse droplets provide homogeneous reaction conditions. It can be combined with cell sorting tools such as Fluorescence Activated Cell Sorting (FACS), to achieve higher speed and efficiency compared to conventional approaches. Benefits include:
- Lower costs while increasing sequencing accuracy and depth:
- Sorting and sequencing only cells containing droplets could vastly reduce reagent costs and increase accuracy and depth for downstream Next Generation Sequencing, including eliminating common issues with single-cell droplet sequencing such as reads from empty droplets due to the encapsulation of free-floating transcripts.
- Increased range of measurement:
- Encapsulated cells may be sorted based on phenotypes not currently measurable with standard cell sorting, such as enzymatic turnover, presence of secreted molecules, or quantification of proteins lacking cell surface markers.
- Multi-omics profiling:
- In contrast with conventional methods, cell sorting using microfluidics provides a unique tool to link genotypes with phenotypes through compartmentalization.
- Our cell encapsulation platform allows for the combination of single-cell phenotyping and genome-wide sequencing, enabling multiomic measurements and directly linking cellular phenotypes to their underlying genetic mechanism2.
E. Mastrobattista, V. Taly, E. Chanudet, P. Treacy, B. T. Kelly, A. D. Griffiths, Chemistry & Biology 2005, 12, 1291.
Drug delivery
Cell or drug encapsulation for drug delivery consists of the immobilization of bioactive materials, mainly cells, within a double emulsion, generally surrounded by a polymeric membrane. The latter permits the free passage of nutrients and oxygen and the egress of therapeutic protein products. Encapsulation allows for the protection of the cell content from mechanical stress and in the case of allogeneic tissue also from the host’s immune response. Microcapsules are commonly used in pharmaceutical or medical processes as drug carriers or for the encapsulation of organic cells.
Locally-implanted droplets may represent a minimally invasive manner of delivering therapeutic proteins such as growth factors and cytokines. It could also allow for mosaic injections containing several droplets to provide complex release profiles or multi-protein deliveries3.
Transplants
The microencapsulation of living cells may serve as an alternative therapy for patients requiring organ transplants. Researchers can control droplets size to mitigate the immune infiltration of transplanted cells while keeping oxygen and waste products transported. Droplets can also immobilize cells at the desired transplant size, which is essential as many cell therapies rely on systemic cell administration.
Cell encapsulation for cell-based therapy is emerging as a promising strategy for treating a wide range of human diseases, such as diabetes, blood disorders, acute liver failure, spinal cord injury, and several types of cancer through transplants. Pancreatic islets, blood cells, hepatocytes, and stem cells are among the many cell types currently used for this strategy. The encapsulation of these “therapeutic” cells is intended to prevent immune rejection, to provide a controlled and supportive environment, and to enable a complete retrieval of the graft in the case of an adverse body reaction4.
Specifications
We propose 4 different platforms to target different droplet sizes.
| Description | Product | P.N |
| Cell encapsulation device | Cell encapsulation platform | O-FACS1-PTF |
| Double Emulsion production device | – RayDrop Double Emulsion 30-70-45 | – O-DE-RDRPC05- EUP |
| Fluid handling system | – LINK Module – Flow EZ 7 bars for all 3 phases | – LU-LNK-0002 – LU-FEZ-7000PCK |
| Reservoirs | – Continuous phase: 50 mL Pcap with 50 mL Falcon tube Tubing: 500 µm – Shell phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm – Core phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm | – P-CAP50-HP-PCK – P-CAP15-HP-PCK – P-CAP15-HP-PCK |
| Flow Meters | – Continuous phase: Flow Unit L – Shell phase: Flow Unit M – Core phase: Flow Unit M | – FLU-L-D – FLU-M-D – FLU-M-D |
| Optical System | – Light source – Microscope objective (x10) – Specific colour camera (up to 400 fps, 1µs integration time) – XYZ translation stages | N/A |
| Tubing & Fittings | – Tubing: OD: 1/16 and 1/32 OD ID: 250 µm & 500 µm Materials: PFA – Manual valves: 4 way valves 2 way valves – Filters: 2 µm filter for continuous phase 2 µm filter for dispersed phase | N/A |
| Wetted materials | – Platform: PEEK, PFA, PCTFE, PTFE, SS316L, GLASS – Sealing: FFKM | N/A |
| Unit dimensions | – 61 x 46 x 43cm 3 (L x W x H) | N/A |
| Weight | – 4 kg without the protective wood – 21 kg with the protective wood | N/A |
| Droplet Size Range* | 25-45 µm | N/A |
| Description | Product | P.N |
| Cell encapsulation device | Cell encapsulation platform | O-FACS2-PTF |
| Double Emulsion production device | – RayDrop Double Emulsion 30-70-60 | – O-DE-RDRPC06- EUP |
| Fluid handling system | – LINK Module – Flow EZ 7 bars for all 3 phases | – LU-LNK-0002 – LU-FEZ-7000PCK |
| Reservoirs | – Continuous phase: 50 mL Pcap with 50 mL Falcon tube Tubing: 500 µm – Shell phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm – Core phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm | – P-CAP50-HP-PCK – P-CAP15-HP-PCK – P-CAP15-HP-PCK |
| Flow Meters | – Continuous phase: Flow Unit L – Shell phase: Flow Unit M – Core phase: Flow Unit M | – FLU-L-D – FLU-M-D – FLU-M-D |
| Optical System | – Light source – Microscope objective (x10) – Specific colour camera (up to 400 fps, 1µs integration time) – XYZ translation stages | N/A |
| Tubing & Fittings | – Tubing: OD: 1/16 and 1/32 OD ID: 250 µm & 500 µm Materials: PFA – Manual valves: 4 way valves 2 way valves – Filters: 2 µm filter for continuous phase 2 µm filter for dispersed phase | N/A |
| Wetted materials | – Platform: PEEK, PFA, PCTFE, PTFE, SS316L, GLASS – Sealing: FFKM | N/A |
| Unit dimensions | – 61 x 46 x 43cm 3 (L x W x H) | N/A |
| Weight | – 4 kg without the protective wood – 21 kg with the protective wood | N/A |
| Droplet Size Range | 45-60 µm | N/A |
| Description | Product | P.N |
| Cell encapsulation device | Cell encapsulation platform | O-FACS3-PTF |
| Double Emulsion production device | – RayDrop Double Emulsion 60-120-60 | – O-DE-RDRPC07- EUP |
| Fluid handling system | – LINK Module – Flow EZ 7 bars for all 3 phases | – LU-LNK-0002 – LU-FEZ-7000PCK |
| Reservoirs | – Continuous phase: 50 mL Pcap with 50 mL Falcon tube Tubing: 500 µm – Shell phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm – Core phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm | – P-CAP50-HP-PCK – P-CAP15-HP-PCK – P-CAP15-HP-PCK |
| Flow Meters | – Continuous phase: Flow Unit L – Shell phase: Flow Unit M – Core phase: Flow Unit M | – FLU-L-D – FLU-M-D – FLU-M-D |
| Optical System | – Light source – Microscope objective (x5) – Specific colour camera (up to 400 fps, 1µs integration time) – XYZ translation stages | N/A |
| Tubing & Fittings | – Tubing: OD: 1/16 and 1/32 OD ID: 250 µm & 500 µm Materials: PFA – Manual valves: 4 way valves 2 way valves – Filters: 2 µm filter for continuous phase 2 µm filter for dispersed phase | N/A |
| Wetted materials | – Platform: PEEK, PFA, PCTFE, PTFE, SS316L, GLASS – Sealing: FFKM | N/A |
| Unit dimensions | – 61 x 46 x 43cm 3 (L x W x H) | N/A |
| Weight | – 4 kg without the protective wood – 21 kg with the protective wood | N/A |
| Droplet Size Range | 50-60 µm | N/A |
| Description | Product | P.N |
| Cell encapsulation device | Cell encapsulation platform | O-FACS4-PTF |
| Double Emulsion production device | – RayDrop Double Emulsion 60-120-90 | – O-DE-RDRPC08- EUP |
| Fluid handling system | – LINK Module – Flow EZ 7 bars for all 3 phases | – LU-LNK-0002 – LU-FEZ-7000PCK |
| Reservoirs | – Continuous phase: 50 mL Pcap with 50 mL Falcon tube Tubing: 500 µm – Shell phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm – Core phase: 15 mL Pcap with 15 mL Falcon tube Tubing: 125 µm | – P-CAP50-HP-PCK – P-CAP15-HP-PCK – P-CAP15-HP-PCK |
| Flow Meters | – Continuous phase: Flow Unit L – Shell phase: Flow Unit M – Core phase: Flow Unit M | – FLU-L-D – FLU-M-D – FLU-M-D |
| Optical System | – Light source – Microscope objective (x5) – Specific colour camera (up to 400 fps, 1µs integration time) – XYZ translation stages | N/A |
| Tubing & Fittings | – Tubing: OD: 1/16 and 1/32 OD ID: 250 µm & 500 µm Materials: PFA – Manual valves: 4 way valves 2 way valves – Filters: 2 µm filter for continuous phase 2 µm filter for dispersed phase | N/A |
| Wetted materials | – Platform: PEEK, PFA, PCTFE, PTFE, SS316L, GLASS – Sealing: FFKM | N/A |
| Unit dimensions | – 61 x 46 x 43cm 3 (L x W x H) | N/A |
| Weight | – 4 kg without the protective wood – 21 kg with the protective wood | N/A |
| Droplet Size Range | 70-90 µm | N/A |
OxyGEN
| Control in real-time, protocol automation, data record and export |
| ver. 2.2.0.0 or more recent |
Software Development Kit
| Custom software application |
| ver. 22.2.0.0 or more recent |
References
- Steele, J.A.M. et al. (2014) “Therapeutic cell encapsulation techniques and applications in diabetes,” Advanced Drug Delivery Reviews, 67-68, pp. 74–83. Available at: https://doi.org/10.1016/j.addr.2013.09.015.
Collins, D.J. et al. (2015) “The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation,” Lab on a Chip, 15(17), pp. 3439–3459. Available at: https://doi.org/10.1039/c5lc00614g.Headen, D.M., García, J.R. and García, A.J. (2018) “Parallel droplet microfluidics for high throughput cell encapsulation and synthetic microgel generation,” Microsystems & Nanoengineering, 4(1). Available at: https://doi.org/10.1038/micronano.2017.76.Farina, M. et al. (2019) “Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond,” Advanced Drug Delivery Reviews, 139, pp. 92–115. Available at: https://doi.org/10.1016/j.addr.2018.04.018.- Damiati, S. et al. (2018) “Microfluidic devices for drug delivery systems and drug screening,” Genes, 9(2), p. 103. Available at: https://doi.org/10.3390/genes9020103.
Expertise & resources
-
Microfluidic Application Notes A quick and efficient double encapsulation method for FACS-based droplet sorting Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes Encapsulation of Cells In Small Double Emulsions Read more
-
Fluigent products manual Cell Encapsulation Platform User’s Manual Download
-
Fluigent Products Datasheets Cell encapsulation platform Datasheet Download
-
Fluigent Products Datasheets FLOW UNIT+ Datasheet Download
-
Microfluidic Application Notes Encapsulation of multiple emulsions in a single droplet Read more
-
Microfluidic Application Notes PLGA microcapsules synthesis Read more
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Expert Reviews: Basics of Microfluidics The Raydrop | A new droplet generation device based on non-embedded co-flow-focusing Read more
-
Microfluidic Application Notes Double Emulsion Generation Read more
-
Microfluidic Application Notes Microfluidic Chitosan Microcapsules Production Read more
-
Microfluidic Application Notes UV-Crosslinking of Microcapsules Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Droplet Production Method Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
-
Fluigent Products Datasheets Flow EZ™ Datasheet Download