Contamination-free, sterile
High Throughput Cell Perfusion Pack
[OOC-ST-001]The most efficient system for creating high throughput cell perfusion
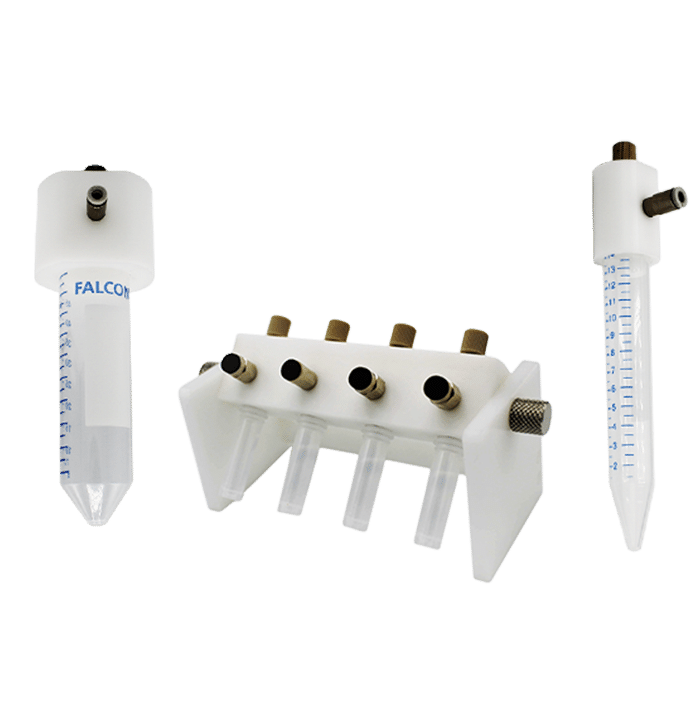
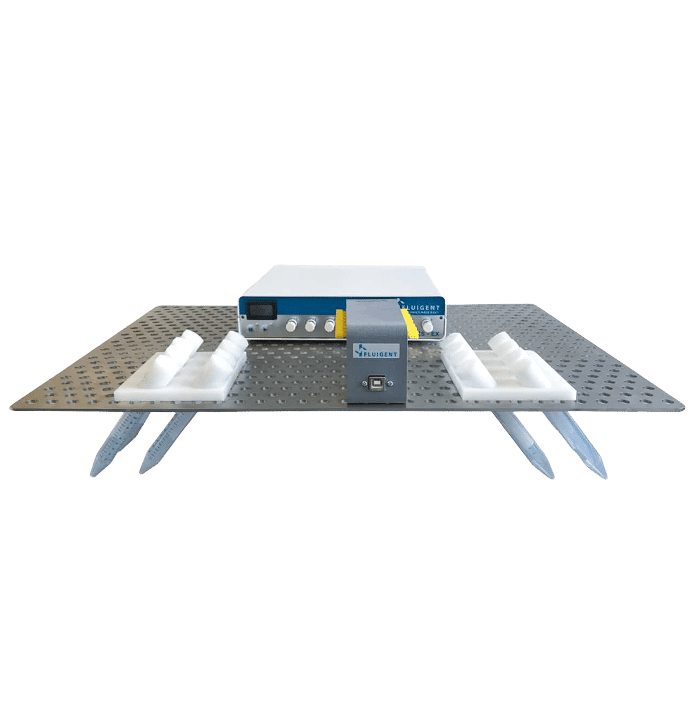
Our cell perfusion pack is designed for high throughput experiments and optimized to perfuse chips while growing multiple organ models in an incubator. It includes an 8-channel compact pressure controller, a flow rate platform, and reservoir holders directly adapted on an incubator trail.
This setup features an intuitive interface that mimics physiological conditions to perform long-term experiments without additional effort while also ensuring high reproducibility and scalability.
- Designed for cell biology
- Easy to use
Simple connections
- Multiplexing
Designed for experiments parallelization
What’s included in the pack
- Fluigent’s MFCS-EX: A microfluidic flow controller with 8 customizable channels with different pressure ranges for high precision operations in microfluidic experiments.
- Fluigent’s Flow Unit : Bidirectional microfluidic flow sensors available in multiple low-rate ranges – up to 8 flow units can be used in one platform.
- Incubator grid: An on-demand, customizable grid compatible with the incubator along with reservoir holders.
- Fluigent connectors and tubing kit.
Features of the cell perfusion system
Stable & Complex Flow Patterns
Fluigent products have unprecedented performances in terms of precise control, stability,and responsiveness. High stability is particularly useful for applications with constant shear stress, like vascular perfusion.
Our Cell Perfusion Pack creates high responsiveness in the reproduction of complex flow patterns such as aortic pressure variations. This level of control ensures consistent and reproducible experimental conditions, minimizing experimental variability.
Protocol automation & user-friendly interface
Once the parameters are set and optimized, protocol automation is the key to saving time, limiting contamination, and decreasing variability. Using any protocol, valves, or pressure, Fluigent flow controllers can be assembled to automate protocols using user-friendly software (OxyGEN).
Researchers can quickly become proficient in using the system, reducing the learning curve, and maximizing productivity.
Versatility
This package can be used for diverse applications with any microfluidic chip.
Enhanced High-Throughput Capabilities
With the ability to run multiple assays in parallel, our perfusion pack is specifically designed to accommodate high-throughput experimentation while efficiently generating data. Compatible with Various Applications
The package is versatile and suitable for a wide range of applications, including drug screening, disease modeling, toxicity testing, and personalized medicine studies.
Customization
Our high throughput perfusion pack is flexible and adaptable to various organ-on-a-chip models and research needs (specific flow rates, pressure, etc.) The cell perfusion package’s modularity allows researchers to design experiments tailored to their unique research questions.
Related applications
Microfluidic perfusion for cell culture
Microfluidictechnology has revolutionized cell culture systems by enabling precise control over fluid flow and creating microenvironments that mimic physiological conditions. One key technique in microfluidics is cell perfusion, which involves the controlled flow of fluid over cells or tissues to maintain their viability and functionality.
Cell perfusion begins with the design of a microfluidic device that incorporates intricate channels or networks, typically on a micrometer scale. Cells are then seeded onto the device, either as a monolayer or in 3D structures, and a continuous flow of culture media or other desired fluids is established within the device. This flow is carefully regulated by a pressure-based controller to provide nutrients, remove waste products, and maintain a stable microenvironment.
The ability to precisely control the flow rate and duration of perfusion allows researchers to mimic physiological conditions, such as blood flow rates in blood vessels. Real-time monitoring and analysis of parameters like cell viability, proliferation, and metabolic activity provide valuable insights into cellular behavior within these microenvironments.
What are possible applications for our cell perfusion pack?
Drug Screening and Development
This setup facilitates the testing of potential drug candidates under more realistic physiological conditions. High-throughput capabilities enable the screening of numerous drug compounds simultaneously, accelerating the drug discovery process and reducing costs.
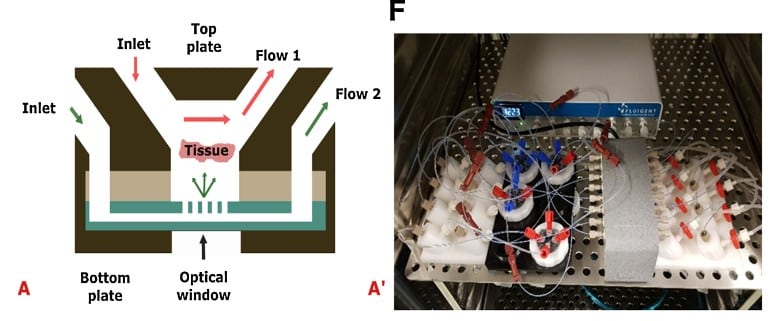
In this example of the application, Chakrabarty et al. (1) developed a novel microfluidic Cancer-on-a-Chip platform to evaluate patient treatment responses using controlled growth conditions for tumor tissue slices. This system allows for the effective prediction of treatment responses for breast and prostate tumor models, and the culture period could be extended up to 14 days without significant changes in tissue quality.
Disease Modeling and Pathophysiology Studies
By investigating cellular responses to disease-related factors, this cell perfusion pack could provide valuable insights into disease pathophysiology, facilitating the development of targeted therapies.
In this example, Messelmani et al. (2) developed a new liver-on-a-chip model integrating a hydroscaffold, allowing cells to organize into complex 3D spheroid architecture. This model could play a role in producing a promising device for disease modeling, drug screening,and risk assessment.
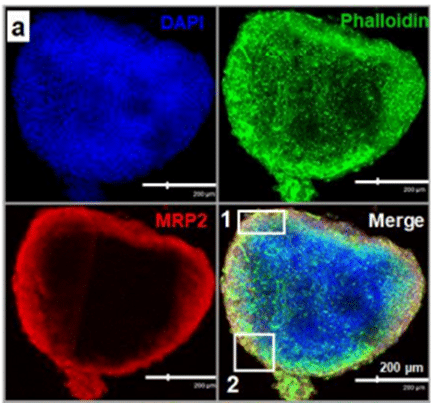
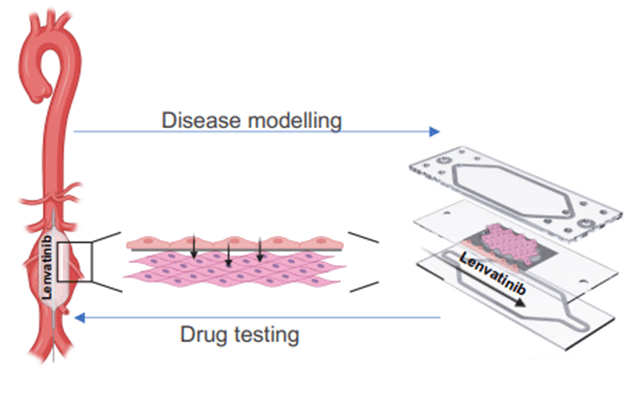
Another example of disease modeling using Fluigent’s MFCS pack is the work of Paloschi et al. (3). They developed an Artery-on-a-Chip model mimicking the arterial vessel wall by incorporating structural aspects of the vasculature (lumen-intima-media) as well as hemodynamic forces impacting the luminal cells. They were able to characterize novel targets previously unrelated to vascular diseases, and to demonstrate that this model system could be used as a platform for testing novel therapeutic agents.
Toxicity Testing and Safety Assessment
High-throughput capabilities of our high throughput cell perfusion pack enable researchers to screen multiple compounds for potential toxicity, helping identify safe and effective substances for further development.
Tissue Engineering and Regenerative Medicine
Researchers can employ the cell perfusion pack to create dynamic microenvironments that promote tissue growth and regeneration.
The platform enables the study of tissue development, cell behavior, and the interactions between different cell types, aiding tissue engineering efforts.
Understanding how tissues respond to different growth factors and conditions can enhance regenerative medicine approaches and tissue transplantation strategies.
Specifications
Fluigent package
| Fluigent’s MFCS-EX (up to 8 channels) |
| Fluigent’s Flow Unit (up to 8 flow unit packed in one platform) |
| Incubator grid (On demand custom grid with reservoir holders) |
| Beonchip microfluidic chip |
| Fluigent connectors and tubing kit |
OxyGEN
| Control in real-time, protocol automation, data record and export |
| ver. 2.2.0.0 or more recent |
Software Development Kit
| Custom software application |
| ver. 22.2.0.0 or more recent |
Expertise & resources
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
-
Microfluidic Application Notes Assess Cell Proliferation Using Pressure as a Tool Read more
-
Microfluidic Application Notes Cartilage-on-a-chip, an example of complex mechanical stimulation using Fluigent’s technology Read more
-
Expert Reviews: Basics of Microfluidics Passive and active mechanical stimulation in microfluidic systems Read more
-
Expert Reviews: Basics of Microfluidics Prostate Organoid Culture in Microbeads Read more
-
Fluigent Products Datasheets FLOW UNIT Datasheet Download
-
Expert Reviews: Basics of Microfluidics Mimicking in-vivo environments: biochemical and biomechanical stimulation Read more
-
Fluigent Products Datasheets MFCS™-EX Datasheet Download
-
Fluigent products manual MFCS™ series User Manual Download
Related products

Microfluidic Flow Control System
MFCS™ series
See the offer
Bidirectional Microfluidic Flow Sensor
FLOW UNIT | FLOW UNIT +
See the offer
Microfluidic cell culture chip
Be-Flow perfused cell culture chip
See the offer
Double channel Microfluidic chip for hypoxic cell culture
BE-DoubleFlow
See the offer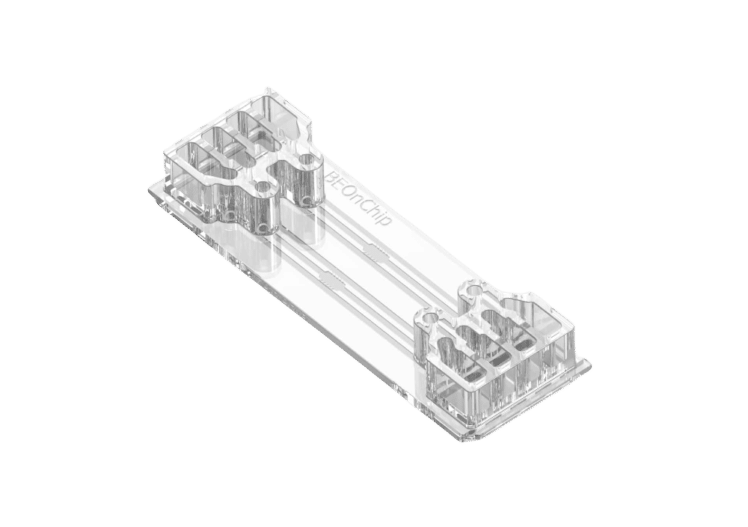
Flow gradient chip for 3D cell cultures
Chip for electrochemical gradients to 3D cell cultures
See the offer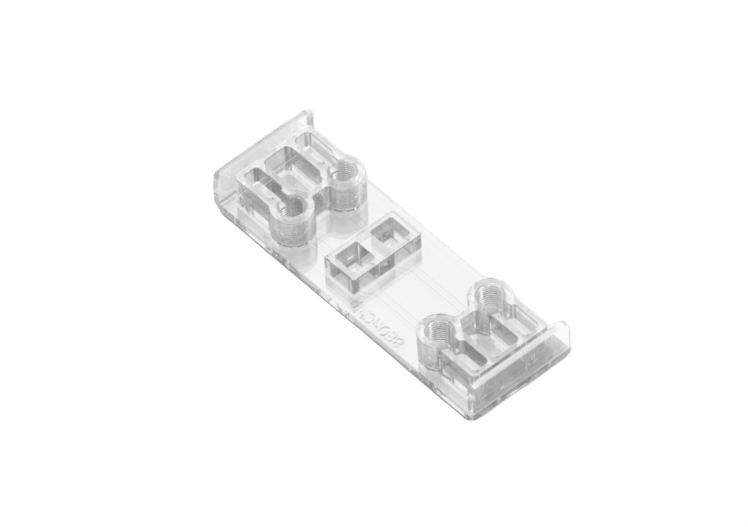
Air Liquid Interface Cell culture versatile chip
Be-Transflow
See the offer
Omi, an Automated Organ-On-A-Chip Platform
Mimic Microphysiological Conditions in Organ-on-a-Chip Studies
See the offer
References:
- Chakrabarty S, Quiros-Solano WF, Kuijten MMP, Haspels B, Mallya S, Lo CSY, Othman A, Silvestri C, van de Stolpe A, Gaio N, Odijk H, van de Ven M, de Ridder CMA, van Weerden WM, Jonkers J, Dekker R, Taneja N, Kanaar R, van Gent DC. A Microfluidic Cancer-on-Chip Platform Predicts Drug Response Using Organotypic Tumor Slice Culture. Cancer Res. 2022 Feb 1;82(3):510-520. doi: 10.1158/0008-5472.CAN-21-0799. Epub 2021 Dec 6. PMID: 34872965; PMCID: PMC9397621.
- Messelmani T, Le Goff A, Souguir Z, Maes V, Roudaut M, Vandenhaute E, Maubon N, Legallais C, Leclerc E, Jellali R. Development of Liver-on-Chip Integrating a Hydroscaffold Mimicking the Liver’s Extracellular Matrix. Bioengineering (Basel). 2022 Sep 5;9(9):443. doi: 10.3390/bioengineering9090443. PMID: 36134989; PMCID: PMC9495334.
- Utilization of an Artery-on-a-chip to unravel novel regulators 2 and therapeutic targets in vascular diseases 3 4 Valentina Paloschi1,2*, Jessica Pauli1,2, Greg Winski3 , Zhiyuan Wu1,4, Zhaolong Li1 5 , Nadiya Glukha1 , Nora Hummel1 , Felix Rogowitz5 , Sandro Meucci6 , Lorenzo Botti7 , Albert Busch1,8 6 , Ekaterina Chernogubova4 , Hong Jin4 , Nadja Sachs1 , Hans-Henning Eckstein1 7 , Reinier A. Boon9,10,11, Andreas R. Bausch12, Lars Maegdefessel1,2,3 https://www.biorxiv.org/content/10.1101/2022.11.29.517312v1