Simple fitting & connections
Flow gradient chip for 3D cell cultures
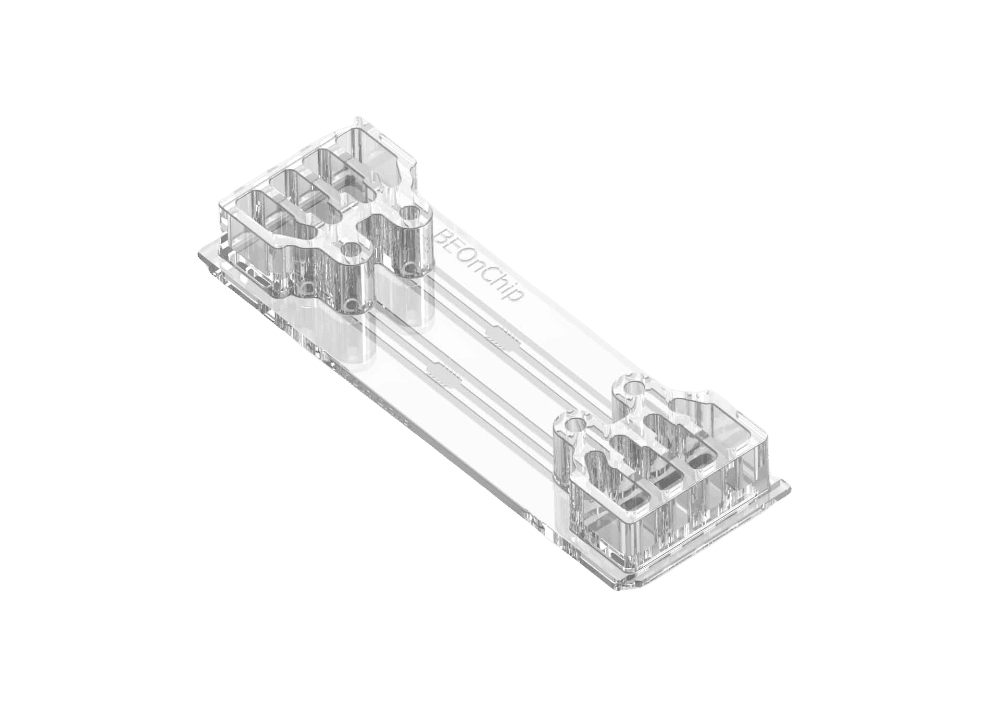
[OOC-GRAD-05]Be-Gradient, flow gradient chip
Designed for the application of electrochemical gradients to 3D cell cultures.
The Flow Gradient chip is Beonchip’s device designed for the application of electrochemical gradients to 3D cell cultures. Be-Gradient is compatible with any type of optical microscopy (inverted phase contrast, confocal, fluorescence..). The chip consists of a central chamber for cell culture and two lateral channels connecting to the central chamber through 3 small microchannels. Lateral channels are meant to simulate blood vessels. 2D culture is also possible for adherent cells not only in the central chamber but also in the lateral channels.
- Easy to use
- Biomimetic environment
2D and 3D cell culture
- SterileDedicated to cell biology
What is a flow gradient microfluidic chip ?
A flow gradient chip is a device that combines microfluidics and electrochemical techniques to create controlled and precise biochemical environments for various applications, such as chemical analysis, cell studies, drug testing, and more.
It utilizes electrochemical reactions to create concentration gradients of specific chemical within the microfluidic channels. A concentration gradient is a gradual change in the concentration of a substance across a distance. This gradient is created by introducing different concentrations of the chemicals at different locations on the chip and allowing them to diffuse and mix within the microfluidic channels.
3D cell culture models with gradients
Apply an electrochemical gradient to your 3D cell culture. First mix your cells in a liquid hydrogel and seed them into the central chamber. After hydrogel polymerization has been completed, perfuse culture media with different concentrations of a chemical compound through the lateral channels and monitor the effect in real time.
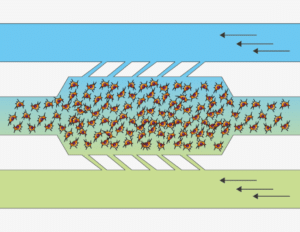
Be-gradient Features
Easy to Use
The chip is compatible with any type of optical microscopy and its slide format has been chosen for easy handling under a microscope.
Easy to Connect
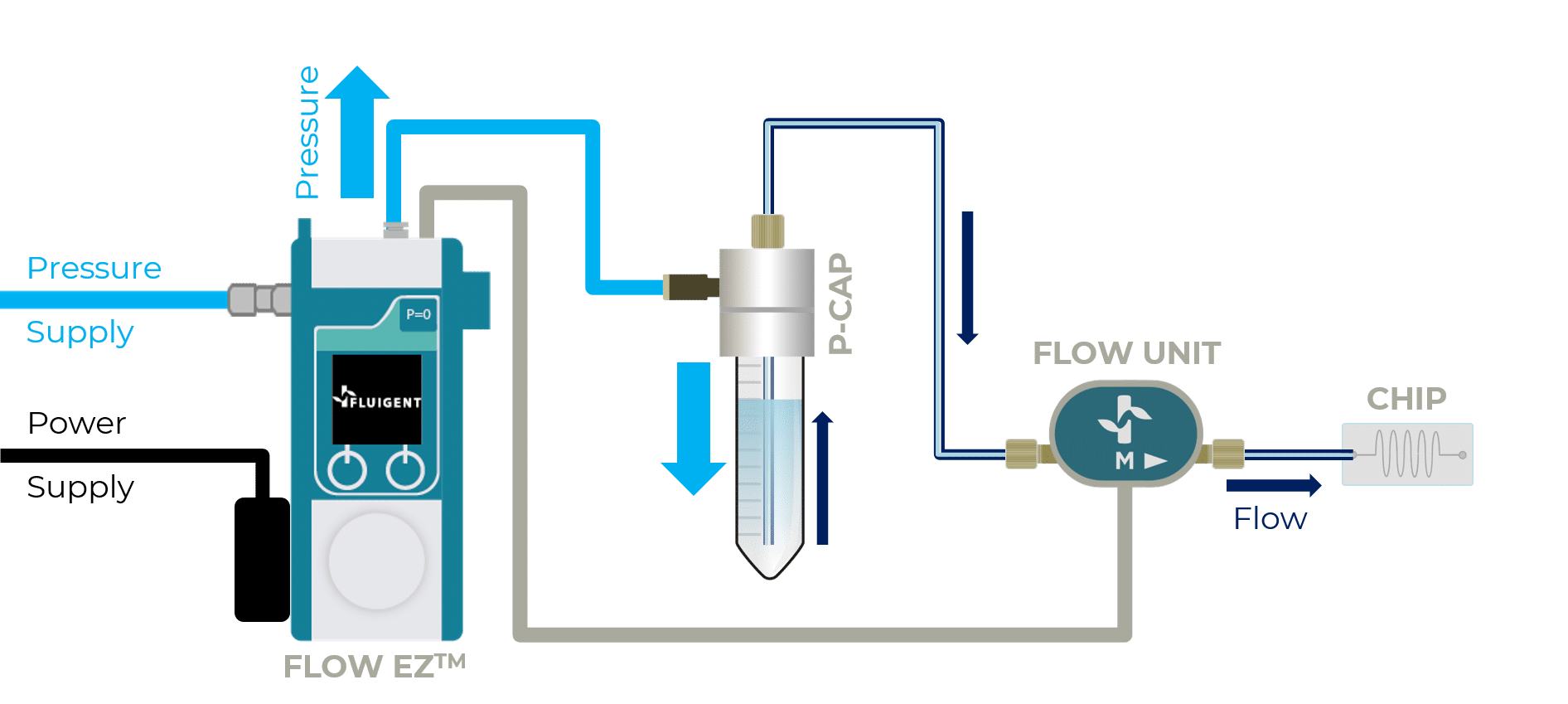
The Beonchip device is compatible with all Fluigent pressure-based flow controllers
No Unspecific Absorption
Unlike in other PDMS devices, Be -Gradient is made of lipophobic thermoplastic materials and does not present unspecific drug absorption issues. It allows immunohistochemistry with fluorescent detection
Cell Recovery
The cell cultures used in the chip can be easily recovered for further experimentation.
Applications with a flow gradient microfluidic chip
The flow gradient chip is a microfluidic chip designed with a very specific function in mind, studying a 3D cell culture under an electrochemical gradient. This device allows experiments that can never be done in a petri dish such as the application of nutrient, oxygen or drug gradient, the study of cell migration under these conditions, angiogenesis studies and much more.
Chemotactic migration studies
The video shows a Multicellular spheroid embedded in collagen and introduced to the microchamber. A gradient of fetal bovine serum (FBS) was established across the central chamber by addition of growth media containing serum into one of the lateral channels.
We observe that spheroids of oral squamous carcinoma cells OSC–19 invade collectively in the direction of the gradient of FBS. This invasion is more directional and aggressive than that observed for individual cells in the same experimental setup. In contrast to spheroids of OSC–19, U87-MG multicellular spheroids migrate as individual cells.
A study of the exposure of spheroids to the chemoattractant shows that the rate of diffusion into the spheroid is slow and thus, the chemoattractant wave engulfs the spheroid before diffusing through it.
The control of the chemotactic gradient across the microchamber, coupled to the ability to observe and closely monitor the system over time, makes BE-Gradient a powerful technique for the study of the chemotactic process. In fact, 3D matrices can mimic in vivo conditions and thus serve as precious tools for cell migration and chemotaxis studies.
In recent years, collective invasion has been proposed as the dominant migration mode during epithelial tumor development. Using the flow gradient chip enables 3D cell culture process to be observed and analyzed in vitro.
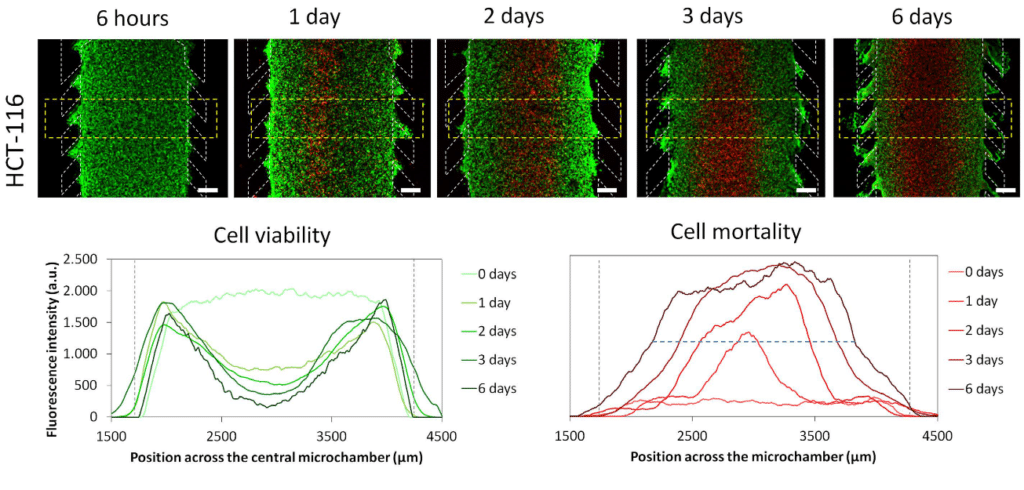
Necrotic core generation within the microdevice
HCT-116 cells were embedded in collagen hydrogel in the central microchamber of the BEOnChip device. 40 million HCT-116 cells/ml were confined in the central microchamber and cell viability was evaluated at the indicated times using calcein (CAM) to stain viable cells green and propidium iodide (PI) to stain dead cells red.
The graphs show CAM or PI fluorescence intensity profile along the delimited region in the images. Position of the pillars is delimited by a grey dashed line. The width of the necrotic core after 6 days was measured as the distance between those positions in the microchamber that reached 50% of the maximum PI fluorescence intensity (blue dashed horizontal line). Necrotic core width was 1643 ± 9 μM, p-value < 0.05. Scale bar is 400 μm.
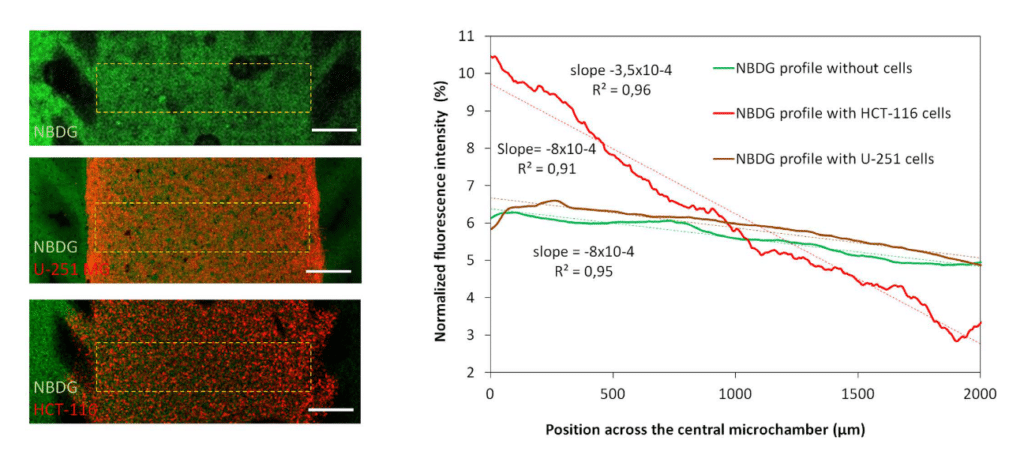
Glucose gradient
Green fluorescent glucose analogue (NBDG, 200 μ M) was perfused through the left lateral channel of the flow gradient microfluidic chip and the diffusion profile was studied in the absence or presence of cells.
The graph shows the NBDG diffusion profile across the central microchamber after 90 min, demonstrating that NBDG was able to penetrate through the collagen hydrogel. The diffusion profile slope was calculated in the absence of cells or in the presence of HCT-116 or U-251 MG cells. Scale bar is 400 μm.
Flow gradient Chip Specifications
Dimensions
| Height | Width | Length | Total volume | |
| Central channel | 300 µm | 1 mm | 39 mm | 12.6 µL |
| Lateral channel | 300 µm | 1 mm | 50 mm | 14.5 µL |
| Chamber | 300 µm | 2 mm | 4.6 mm | 3 µL |
| Inlet/outler | 8 mm | – | 2.3 mm | 18.4 µL |
| Reservoir | 6 mm | 3.6 m | 7 mm | 151.2 µL |
3D cell culture with gradients
Getting Started
Expertise & resources
-
Microfluidic Application Notes Development of a human gut-on-chip to assess the effect of shear stress on intestinal functions Read more
-
Expert Reviews: Basics of Microfluidics How to choose a microfluidic chip Read more
-
Fluigent Products Datasheets BE-Gradient datasheet Download
-
Expert Reviews: Basics of Microfluidics Passive and active mechanical stimulation in microfluidic systems Read more
-
Expert Reviews: Basics of Microfluidics Mimicking in-vivo environments: biochemical and biomechanical stimulation Read more
Related products

Microfluidic flow controller
Flow EZ™
See the offer
Bidirectional Microfluidic Flow Sensor
FLOW UNIT | FLOW UNIT +
See the offer
Automated Perfusion System for Spatial Omics
Aria
See the offer
Microfluidic cell culture chip
Be-Flow perfused cell culture chip
See the offer
Double channel Microfluidic chip for hypoxic cell culture
BE-DoubleFlow
See the offer
Air Liquid Interface Cell culture versatile chip
Be-Transflow
See the offer
Omi, an Automated Organ-On-A-Chip Platform
Mimic Microphysiological Conditions in Organ-on-a-Chip Studies
See the offer
High Throughput Cell Perfusion Pack
High Throughput Cell Perfusion Pack
See the offer