Human Blood Brain Barrier (BBB) permeability -on-chip assessment
Roger D. Kamm’s group at MIT (Massachusetts Institute of Technology) has developed a microfluidic BBB permeability model of the human blood-brain barrier connected to our Flow-EZ pressure controllers allowing quantitative vascular permeability analyses. Their microfluidic device, recently published in Nature Protocols, offers a human BBB model in terms of vascular morphology and appropriate cellular organization, transport capabilities and relevant gene/protein expression profiles.
Cynthia Hajal, Giovanni S. Offeddu, Yoojin Shin, Shun Zhang, Olga Morozova, Dean Hickman, Charles G. Knutson & Roger D. Kamm
Nat Protoc 17, 95–128 (2022). https://doi.org/10.1038/s41596-021-00635-w
Introduction
What is Blood-Brain Barrier permeability?
The Blood-brain barrier (BBB) is a network of vessels that acts as a strong barrier that separates blood from the brain’s fluid in the CNS. It is made of specialized endothelial cells in these vessels that prevent most substances from freely moving in and out of the brain.
The BBB controls what can enter the brain from the bloodstream. The blood-brain barrier permeability determines how easily substances can pass through to reach the brain.
It is highly selective and tightly regulated. Its main job is to keep the brain safe from things that could be harmful, while letting important nutrients and molecules go through. This helps maintain a stable environment for the brain and protects it from harmful substances.
What impact the Blood-Brain Barrier permeability?
The BBB’s permeability is influenced by various factors, such as neurons and extracellular matrix, that work together to regulate its function.
Under normal conditions, the BBB prevents the passage of bacteria, large molecules, and most small molecules into the brain. To enter the brain, substances need to be small, lipid-soluble, and not actively transported out. Some substances can cross using specific transport mechanisms. However, in certain situations like inflammation or injury, the BBB’s integrity can be compromised, allowing larger and more hydrophilic substances to get through.
BBB permeability can vary depending on several factors:
- Size and structure of molecules: Small, lipid-soluble molecules can go through the BBB more easily than larger, polar molecules which may require specific transport mechanisms.
- Charge: Charged molecules may need specific transporters to cross the BBB due to the barrier’s hydrophobic nature.
- Transport mechanisms: Various transport mechanisms, such as passive diffusion, facilitated diffusion, active transport, and transcytosis, regulate the movement of substances across the BBB. These mechanisms can influence the permeability of molecules.
- Tight junction integrity: The BBB is composed of endothelial cells that are linked tightly together, creating a barrier that stops substances from moving easily between cells. When the tight junctions in the brain are disrupted, it can increase BBB permeability and allow substances to leak into the brain.
What are the existing BBB models?
In vitro human blood-brain barrier (BBB) models are needed to assess the barrier permeability, pathophysiological molecular transport mechanisms and enable the design of targeted therapies for neurological disorders.
Several 2D culture systems have been designed but often fail to recapitulate the 3D cellular organization of brain capillaries.
The generation of 3D BBB models has allowed the production of tube-like vessels, however their geometries as well as cellular organization are still far from reflecting blood brain barrier in vivo. Here, Hajal et al., developed a human BBB model-on-chip resembling the natural BBB that displays relevant gene expression profiles and vessel permeability values. They also developed quantitative measurement of perfusate molecules.
Experimental procedure
The authors provide a detailed protocol comprising the different steps to fabricate their BBB model as well as methods to quantitatively analyze molecular BBB permeability using the microfluidic technology.
Briefly, PDMS microfluidic chips were molded from a silicon wafer fabricated by soft photolithography. Appropriate cell types such as human endothelial cells (ECs), pericytes (PCs) and astrocytes (ACs) were embedded in a fibrin hydrogel and loaded inside the microfluidic chip (Figure 1).
As a result of cell self-organization, microvascular networks (MVNs) were formed after few days of culture within the chips.
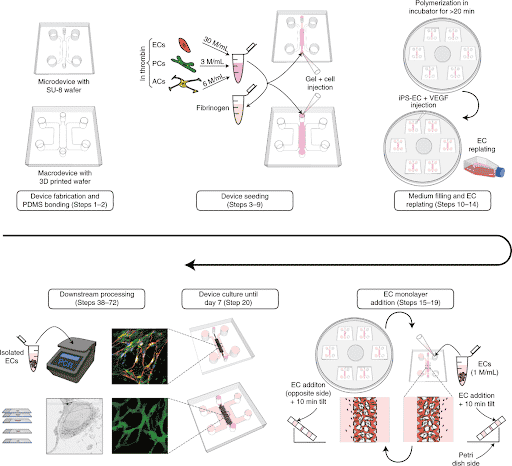
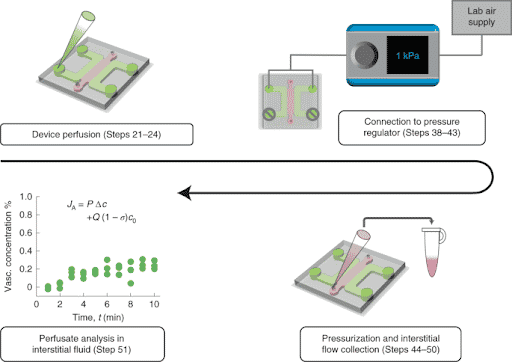
In addition, the authors develop an approach to precisely collect interstitial fluid for direct analysis of labeled and unlabeled molecules. They used Fluigent Flow-EZ pressure controllers to perfuse their Brain Blood Barrier model by imposing a pressure of 1kPa and further collected interstitial fluid (Figure 2). This controlled fluid perfusion and collection enable to analyze molecules present in this fluid with methods such as ELISA or mass spectrometry.
These two methods (interstitial fluid collection following vascular pressurization and fluorescence imaging by confocal microscopy) can be combined to measure effective blood-brain barrier permeability values under physiological transmural flow conditions.
This robust and flexible method could be applied to numerous applications. Analyzing the BBB permeability to nanoparticles for instance or the susceptibility to infectious agents such as SARS-CoV-2 would be of great interest.
Results
Compared with standard 2D assays, this BBB model features relevant cellular organization and morphological characteristics, as well as values of molecular permeability within the range expected in vivo. After several days of culture, highly interconnected structures are formed (Figure 3) with vascular diameters in the range of 10–40 µm (slightly larger than those of human BBB capillaries in vivo).
They can be perfused with solutes which make it a highly physiologically relevant permeability model microcirculation. Importantly, the application of physiological levels of fluid flow using a microfluidic pump was previously shown to induce lower permeabilities and resulted in prolonged model stability (Gs, O. et al. Microheart: a microfluidic pump for functional vascular culture in microphysiological systems. J. Biomech. 119, 2021).
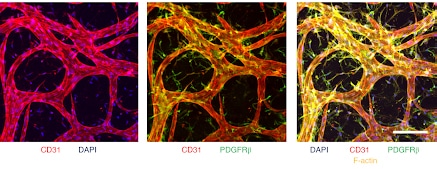
Microvascular networks formed inside the Blood Brain Barrier-on-chip can be collected from the gel to be analyzed. Cell types such as endothelial cells can be isolated with a cell sorter to quantify their gene and protein expression levels. The results show that under appropriate culture conditions with BBB-specific perivascular cells (pericytes and astrocytes), human ECs (ECs from induced pluripotent stem cells = iPS-ECs) adopt gene expression profiles that closely match those of human primary brain ECs.
In addition, the assessment of the permeability of various molecules as well as the analyses of circulating cytokines secreted in the BBB-on-chip (via Luminex analysis of the medium perfused) provides a simple and physiologically accurate platform to study correlations between cytokine signaling and transporter gene/protein expression.
Furthermore, the relevance of this protocol in designing patient-specific Blood Brain Barrier microvascular networks may have potential applications in the clinic.
Conclusion
Hajal et al. have established a BBB permeability model-on-chip in which interconnected microvascular networks are formed and that recapitulate key aspects of the natural Blood Brain Barrier. In their protocol, published in the prestigious Nature Protocols journal, our accurate and versatile flow control instrument – Flow-EZ – provides complete control of media perfusion and collection necessary for the analysis of perfusates. The BBB-model-on-chip represents a suitable system for widespread use in academic and industrial laboratories.
Related Resources
- 어플리케이션 노트
Development of a human gut-on-chip to assess the effect of shear stress on intestinal functions
Discover 
White paper: Organ on Chip
Discover- 기술서
Microfluidic white paper – A review of Organ on Chip Technology
Discover - 사용자 사례
CNRS/UTC: study of a liver-on-a-chip model
Discover - 압력 기반 미세유체 제품의 이점
Microfluidic pressure control for organ-on-a-chip applications: A comprehensive guide
Discover - 어플리케이션 노트
Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications
Discover 
WEBINAR – Automated fluid delivery for cell and tissue imaging
Discover
WEBINAR: Enhancing Microfluidic Cell immunolabeling with Aria Technology
Discover