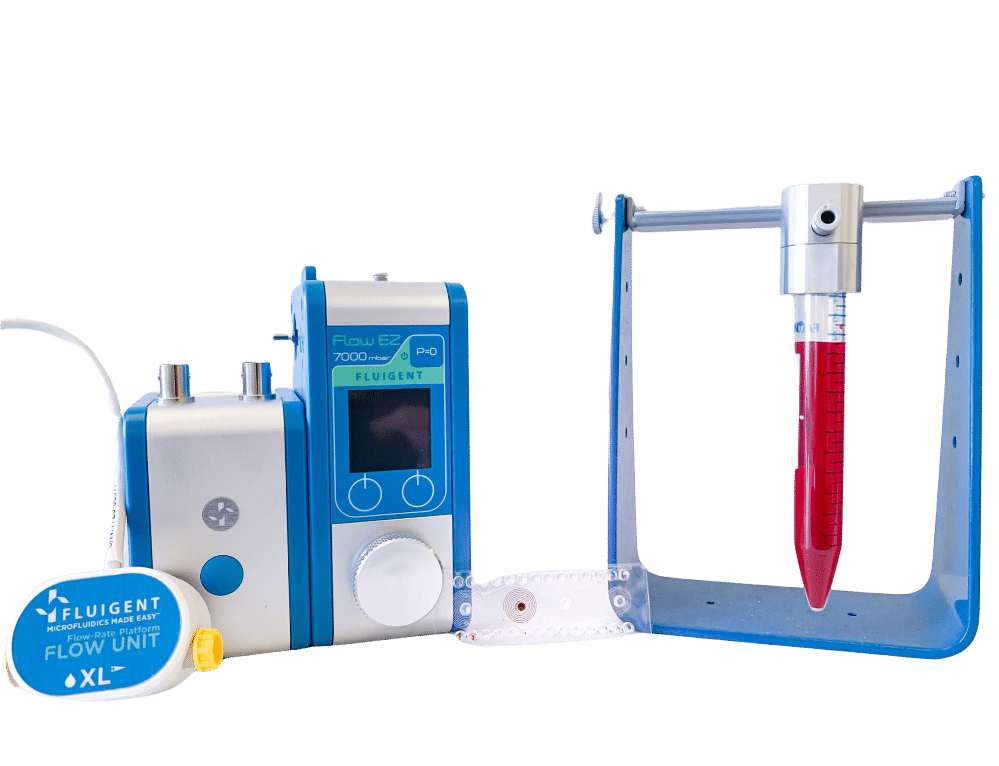
Single cell sorter microfluidic platform
Accurate and high throughput cell sorting is critical for enabling technology in molecular and cellular biology, biotechnology, and medicine. While conventional methods can provide high-efficiency sorting in short timescales, advances in microfluidics have enabled the use of miniaturized devices offering similar capabilities that exploit a variety of physical principles.
Single cell sorter microfluidic platforms provide numerous advantages over conventional methods by reducing the size of necessary equipment, eliminating potentially biohazardous aerosols, and simplifying the complex protocols commonly associated with cell sorting. Additionally, microfluidic chip devices are well suited for parallelization, enabling complete lab-on-a-chip devices for cellular isolation, analysis, and experimental processing (1).
To demonstrate the separation of particle mixtures, a solution containing 7.5 µm and 15 µm diameter polystyrene particles labeled with FITC and TRITC fluorophores was used. The particle streams were viewed and captured separately using appropriate filter cubes.
Sorting of 15 and 7 micrometer diameter bead using a microfluidic platform
Benefits of cell sorting
In many fields of biology, biotechnology, and medicine, isolating and sorting cells from complex, heterogeneous mixtures is a crucial task. Cell sorting is frequently used to enrich or purify cell samples into well-defined populations to increase productivity in research and development applications. Cell sorting also serves as the first step in many diagnostic and therapeutic practices (1).
The need to sort cells is rapidly expanding toward the isolation of rarer target cell populations, including the enrichment of circulating tumor cells (CTCs), hematopoietic stem cells (HSCs), and circulating fetal cells (CFCs) from blood (4).
What is a single cell sorter passive system ?
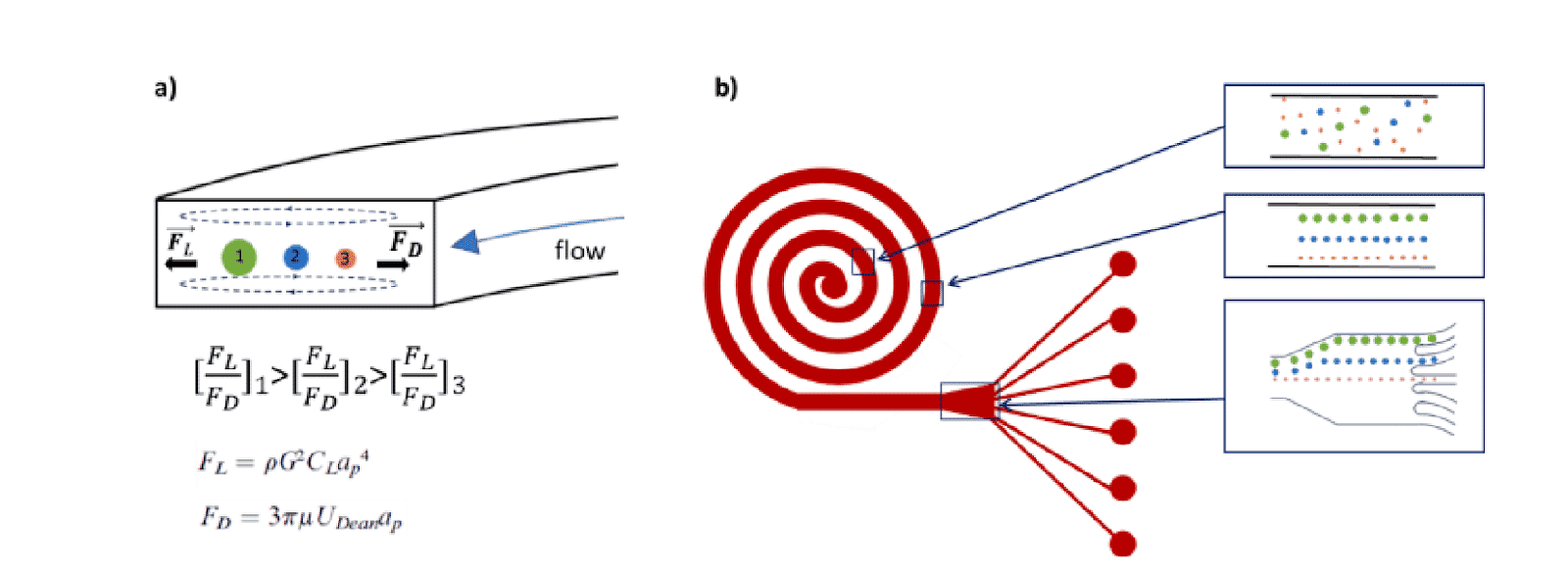
Single cell sorting platform passive systems consist of a variety of methods that do not rely on fluorescent labels or beads. Instead, these methods rely on the inherent differences in cellular morphology between cell groups (e.g., size, shape, compressibility, and density) and can sort cells using inertial forces, hydrodynamic spreading, deterministic lateral displacement, etc.
In a single cell sorter with a passive system, channels with spiral shapes are used to separate particles according to their size based on the Dean forces. The main benefit of this design is high throughput (>1.5 mL/min). Fluigent and microfluidic ChipShop validated an effective and commercial solution for cell sorting.
How to confirme the good seperation of particle mixtures ?
To demonstrate the separation of particle mixtures in the single cell sorting platform, a solution containing 7.5 µm and 15 µm diameter polystyrene particles labeled with FITC and TRITC fluorophore was used. The particle streams were viewed and captured separately using appropriate filter cubes.
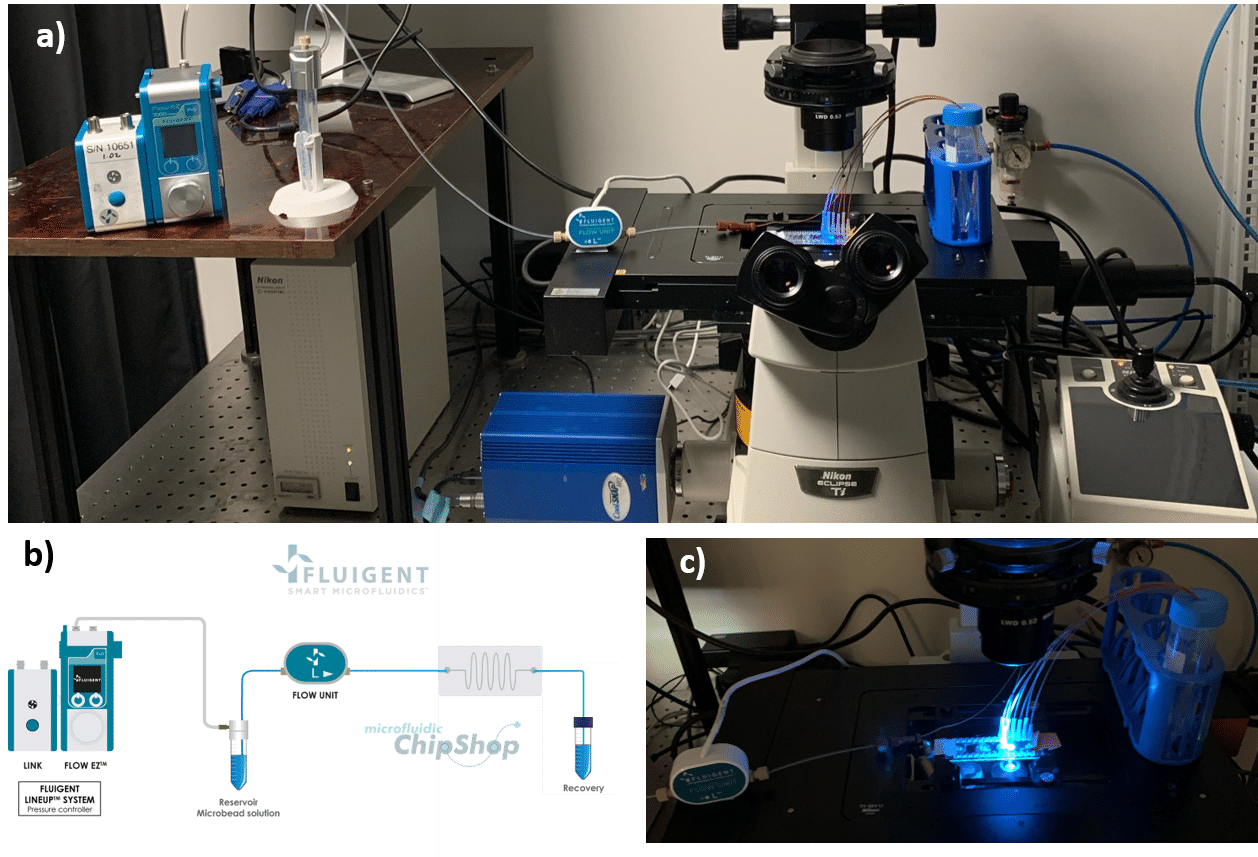
How to perform single cell sorting
Starting sorting experiments – Materials
Reagents:
Fluorescently labelled polystyrene particles purchased from Bangs Laboratories. 7.3 µm (+/- 0.53 µm) with red fluorescence and 15.25 µm (+/- 0.669 µm) with green fluorescence.
Optics
The surfaces are monitored using an inverted microscope (Eclipse Ti-U, Nikon) in bright field or in fluorescence mode. For the acquisition of the fluorescent beads, we use the software NIS-Elements with standard Nikon filter cubes for FITC and TRITC.
What is the method employed to sort single cells?
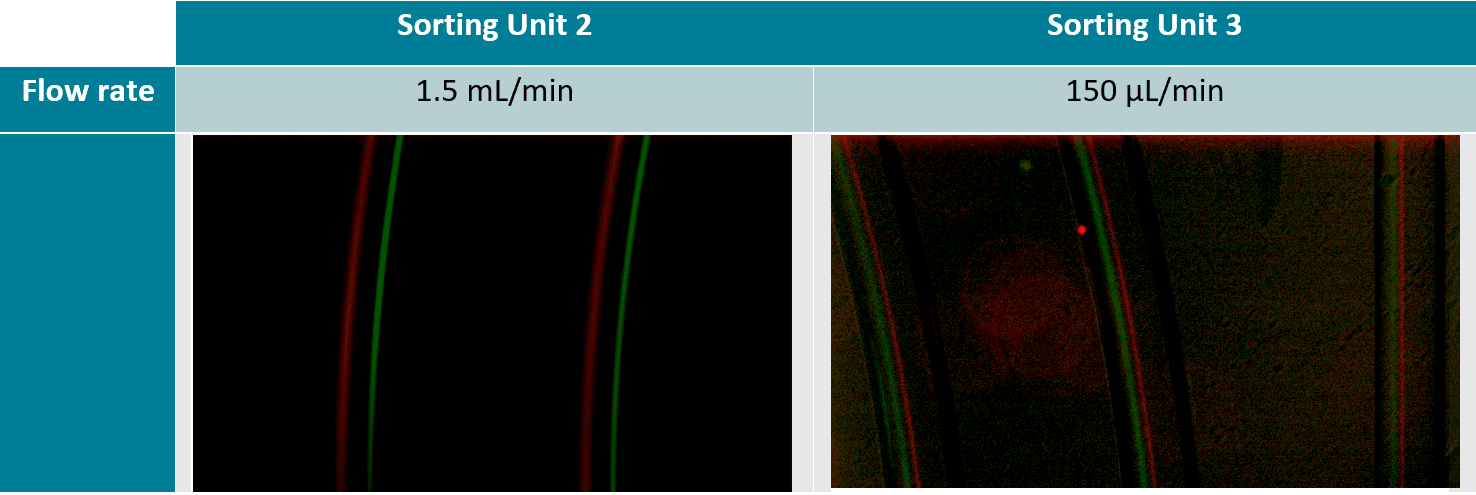
The 7.3 µm and 15.25 µm fluorescent microbeads are mixed and diluted in DI water before testing to reach a concentration of ~1.105 beads/mL. In order to make the single cell sorter, the Flow EZ is connected to the reservoir containing the mixture using a P-CAP. The reservoir is connected to the inlet of the microfluidic device using tubing of 500 µm inner diameter (ID). Tubing passes through the Flow Unit to control and monitor flow rate. Tubing is connected to the outlets of the microfluidic device to recover the beads. The flow rate is set using the Flow EZ. Here, sorting units 2 and 3 with flow rates of respectively 1.5 mL/min and 150 µL/min are used. The particle streams were viewed and captured separately using TRITC and FITC filter cubes.
High-Throughput Separation and Sorting of Fluorescent Microbeads
A homogeneous mixture of 7.3 µm and 15.25 µm fluorescent microbeads are injected into the central inlet of the single cell sorter using flow rates of 1.5 mL/min for unit 2 and 150 µL/min for unit 3. Figure 3 clearly indicates the formation of two distinct particle streams, confirming that 7.3 and 15 µm microbeads are well separated. In addition, as predicted (2) we observe the larger particles (green fluorescence, 15 µm) closer to the inner channel.
In figure 4, the green fluorescence particles are the majority collected at the first and second outlets of the microfluidic device, while red fluorescence particles are collected at the fourth outlet of the same sorting unit.
This result confirms that 7.3 µm and 15 µm particles are well separated and subsequently sorted by the microfluidic device with very high throughput.
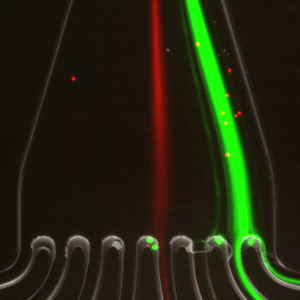
Conclusion
In this application note, we introduced a commercially-available single cell sorter microfluidic system to perform a passive size separation of a microparticle mixture.
The system includes a spiral-shaped microfluidic device from microfluidic ChipShop and pressure-based flow controllers from Fluigent. Tuning Dean and lift forces induced by spiral microchannels allow users to obtain distinct particle streams and subsequently sort particles according to their sizes. Beads with diameters of 15 and 7.3 µm were successfully sorted using flow rates of 150 µL/min and 1.5 mL/min.
We provide an easy to use, versatile, and cost-effective single-cell sorter for particle-size sorting.
Related Resources
WEBINAR: Encapsulation of fluorescent bacteria in double emulsions for FACS sorting
DiscoverWEBINAR: Single cell encapsulations compatible with FACS sorting, API encapsulations in biocompatible polymers, and more
Discover- Microfluidic chips
Application of microfluidic chip technology
Discover - Droplet and particle generation in microfluidics
High Throughput Single Cell Analysis
Discover - Microfluidic cell biology
Micropipette aspiration of cells and tissues
Discover